What is Robinson annulation?
Actually, Robinson annulation is the formation of a ring by following three steps.
(1) First step is Michael addition
(2) Second step is Aldol Condensation
(3) The third step is dehydration.
Annulation means “building of a ring.”
Historical Background:
The British chemist Sir Robert Robinson, who won the 1947 Nobel Prize in Chemistry for his studies on plant products, particularly alkaloids, is remembered by the name of the Robinson annulation reaction. Robinson created this annulation reaction as a result of his work on the synthesis of complicated chemical compounds from nature. This reaction offered a new and effective method of synthesizing six-membered rings, a vital structure of many biologically active compounds.
Michael addition of a ketone enolate to an α,β-unsaturated ketone gives δ-diketone. When the conjugate addition takes place in highly basic or acidic conditions, δ-diketone will spontaneously undertake intramolecular aldol condensation, usually preceded by dehydration, resulting in a six-membered ring known as a conjugate cyclohexanone. Take an example where the Michael donor is a substituted cyclohexanone, and the Michael acceptor is methyl vinyl ketone (MVK).
Robinson Annulation Reaction:
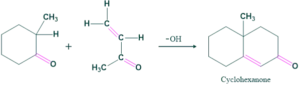
Robinson Annulation Mechanism:
Step (1) Michael’s addition:
When enolate of cyclohexanone is added to MVK, the mechanism starts. First we make enolate by attack of base, and then the enolate acts as a nucleophile. This nucleophile attack on MVK (methyl vinyl ketone) then leads to the formation of δ-diketone.
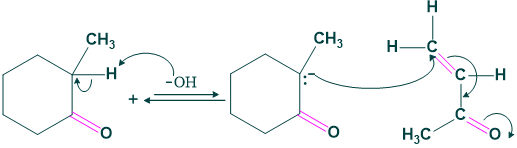
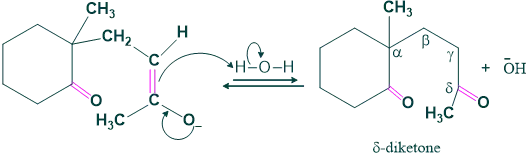
Although this δ-diketone can take part in multiple aldol condensations, it is most suitable for one that is particularly helpful: the synthesis of a six-membered ring. The enolate of methyl ketone attacks the cyclohexanone carbonyl to generate a six-membered ring. After dehydrating, the aldol product yields a cyclohexanone.
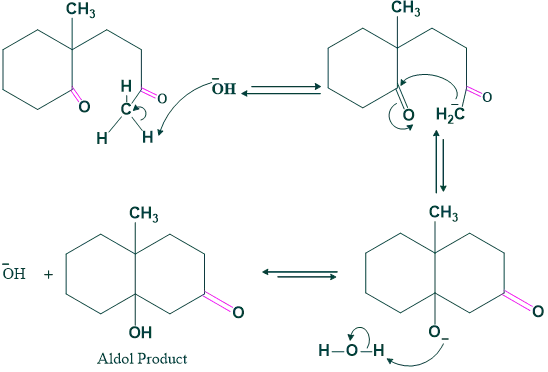
Step (02) Aldol Condensation:
In this step aldol condensation takes place, and here a typical base (OH) attacks a more acidic hydrogen and shows intramolecular cyclization.
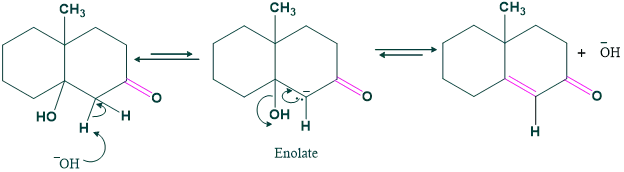
Step (03) Dehydration:
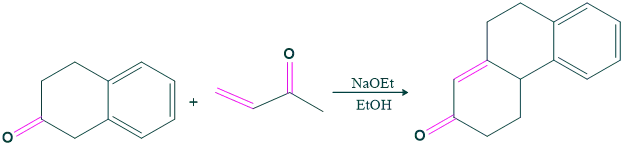
Robinson Annulation Reaction Examples:
Mechanism:
In this step first we form an enolate ion, and then this enolate ion acts as a nucleophile, and an attack on a β-unsaturated carbonyl compound gives a Michael adduct or δ-diketone.
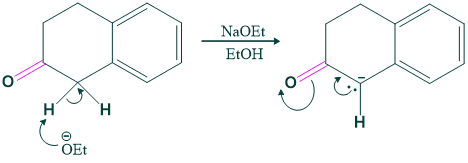
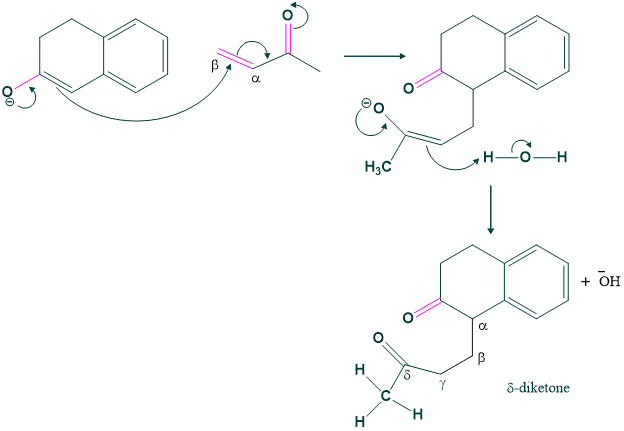
Step (02) Aldol Condensation:
In this step Aldol condensation takes place, and here typically a base (OH) attacks a more acidic hydrogen and shows intramolecular cyclization and gives a product.
Application in Natural Product Synthesis:
Many natural compounds, especially those with six-membered carbocyclic rings, are synthesized via the Robinson annulation. It has been used, for example, in the synthesis of terpenoids, alkaloids, and steroids—classes of natural compounds having significant biological activity.
Steroids:
Three six-membered rings and one five-membered ring make up the four fused rings that make up the steroid nucleus, the central region of steroids. Building the six-membered rings of the asteroid nucleus requires the Robinson annulation.
Alkaloids:
Six-membered rings are present in many alkaloids, including morphine and quinine, and they can be successfully produced by the Robinson annulation. The biological activity of these compounds depends on the chemists’ ability to manufacture these rings with a high degree of control over stereochemistry, which becomes feasible by this reaction.
Terpenoids:
The production of terpenoids, a broad and diverse family of naturally occurring compounds formed from five-carbon isoprene units, is another use for the Robinson annulation. Six-membered rings, which can be created by the Robinson annulation, are found in various terpenoids.