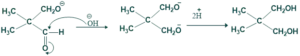
Those carbonyl compound which donot contain α-H react in the presence of conc. base gives alcohol ( reduced product) and acidic salt (oxidized Product).
- It is base induced disproportionation reaction
- Here oxidized product is acid and and reduced product is alcohol
- Here reducing agent show hydride transfer.
Reaction of cannizzaro:
![]()
Cannizzaro Reaction Mechanism:
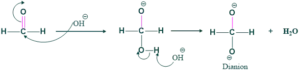
Step(01) formation of dianion:
In this reaction , we use typical base OH which react on less hindered product because our preference is to make acid of that salt which has no steric hindrance . Thus OH attack on formic acid and give dianion.
Step(02) hydride shift:
Here hydride of dianion shifted towards benzaldehyde.
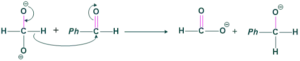
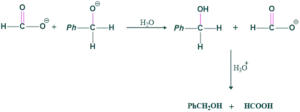
Step(03) Deprotonation of water:
In this step the ions are protonated with water and give respected product.
Types of Cannizzaro Reaction:
- Simple cannizzaro reaction
- Cross Cannizzarro Reaction
Simple Cannizzaro Reaction:
When two same carbonyl compound having no α-H react with each other and give only one product . This type of reaction is called simple cannizarro reaction.
Cannizzaro reaction of benzaldehyde:
![]()
Cannizzaro reaction of formaldehyde:
![]()
Cross Cannizzaro Reaction:
Those reaction in which two different carbonyl compound having no α-H are present react with each other give four different products which as follows;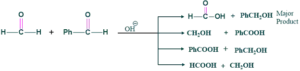
Cross cannizzaro reaction is also called as intramolecular (with in molecule) reaction.
Example No.01:
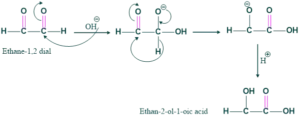
Example No.02:
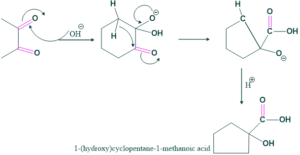
Application of Cannizzaro Reaction:
- Synthesis of Spiro Compound
- Reaction of 2-methyl propanal with formaldehyde
Synthesis of Spiro Compound:
When acetaldehyde and formaldehyde react with each other in the presence of base (OH)-HBr to give bromo compound , which on reduction with zinc/dust give spiro compounds.
Reaction:
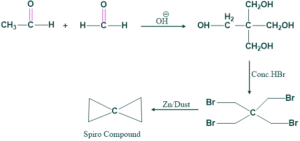
Mechanism:
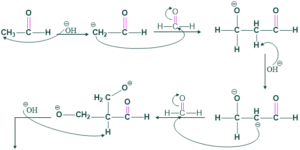
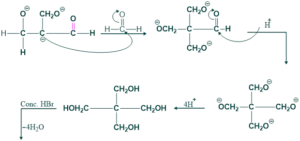
![]()
Reaction of 2-methyl propanal with formaldehyde:
Reaction:
![]()
Mechanism:
Step(01):
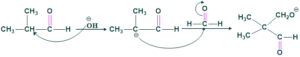
Step(02):
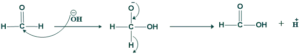
Step(03):