What are Carbohydrates?
It is defined as polyhydroxy (more than one hydroxy group) aldehydes or ketone compounds produced on hydrolysis, which are called carbohydrates. The name carbohydrates means ‘hydrates of carbon.
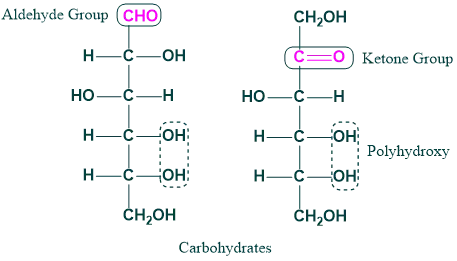
They are the most abundant organic molecules in nature. They are composed of carbon, hydrogen, and oxygen. The empirical formula of carbohydrates is [CH₂O]n.
However, deoxyribose and rhamnohexose do not contain this formula. Some carbohydrates consist of nitrogen, phosphorus, and sulfur.
What is the function of carbohydrates?
- These are sources of energy for all living organisms, e.g., glucose.
- They act as a storage form of energy, e.g., glycogen in animal tissue and starch in plants.
- Carbohydrates (as glycoproteins and glycolipids) participate in the structure of cell membranes and cellular functions such as growth, adhesion, and fertilization. Cellulose in plants, chitin in insects, and glycosaminoglycans in humans.
- Non-digestible carbohydrates, like cellulose, serve as dietary fibers.
- They are also involved in detoxification, e.g., glucuronic acid.
- They play a role in lubrication, cellular intercommunication, and immunity.
- Ribose sugar and deoxyribose sugar contain carbohydrates.
Classification of Carbohydrates:
These are divided into three types:
- Monosaccharides
- Polysaccharides
- Oligosaccharides
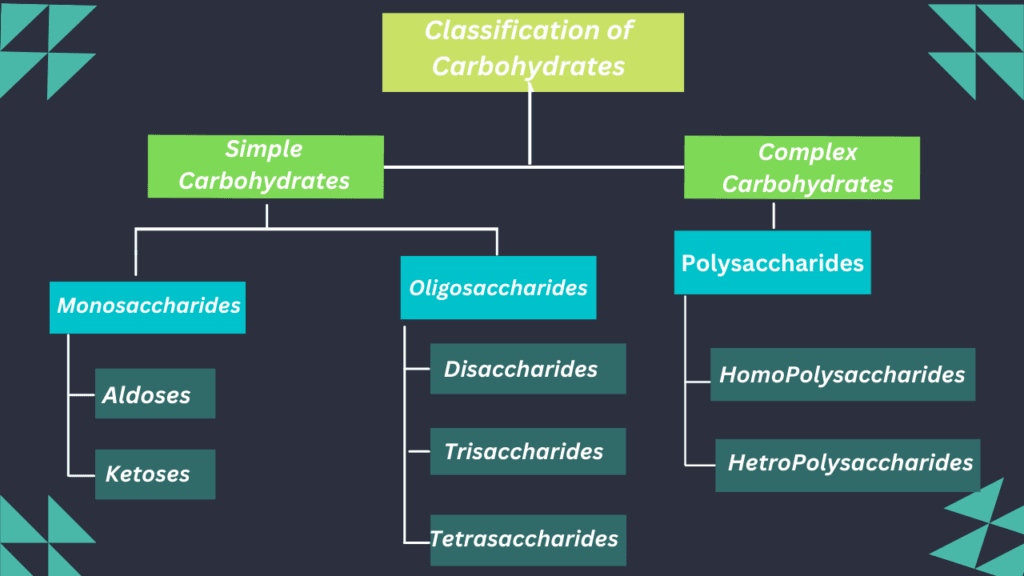
Monosaccharides:
Monosaccharide examples are trioses, tetroses, pentoses, hexoses, heptoses, octoses, and nonoses. It contains an aldehyde functional group called “aldose sugar,” and it contains a ketone functional group in its structure that is called “ketose sugar.” These are the carbohydrates, and they have a general formula (CnH2O)n and cannot be further hydrolyzed.
- They consist of a single polyhydroxy aldehydes and ketone (single unit)
- They are the simplest form of sugar.
- They are soluble in water
- They are sweet
- They cannot be hydrolyzed into further simple sugars.
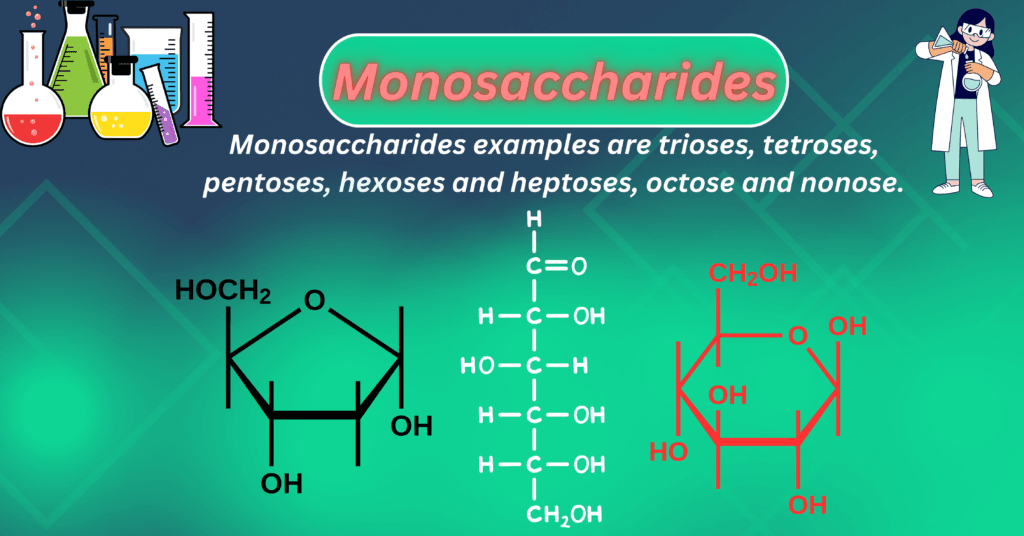
Types of Monosaccharides:
These are two types based on functional groups.
- Aldose Sugar
- Ketose Sugar
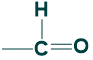
Aldose Sugar:
When the functional group is an aldehyde, it is called aldose sugar
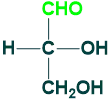
Glyceraldehyde:
Glyceraldehyde is a triose (3C) monosaccharide that contains aldehyde functional groups in its structure. Glyceraldehyde
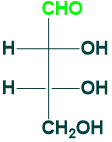
Erythrose:
Erythrose is a tetrose (4C) sugar that contains aldehyde functional groups in its structure. Its 4-phosphate is an intermediate in carbohydrate metabolism.
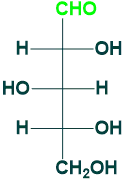
Ribose:
Ribose is (5C) aldose sugar also known as pentose sugar.
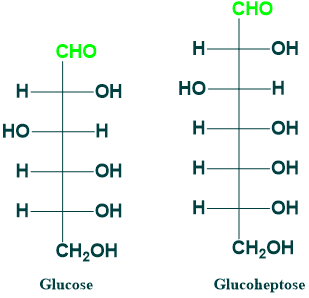
Glucose and Glucoheptose:
Glucose is (6C) aldose sugar While glucoheptose is a (7C) aldose sugar.
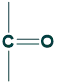
Ketoses Sugar
When a functional group is a ketone, it is called a ketose sugar.
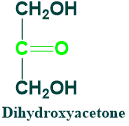
Dihydroxyacetone:
It is also known as triulose.
It is found in cells as a phosphate
Its 1-phosphate is an intermediate in glycolysis
It is 3C ketose sugar
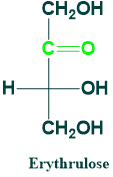
Erythrulose:
It is a 4C ketose sugar. It is also known as cellulose.
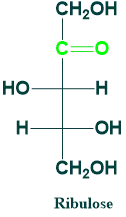
Ribulose
It is also known as pendulous.
It is a 5C ketose sugar.
It is produced during metabolism.
It is an important metabolite in the hexose monophosphate shunt.
Fructose
It is also known as hexulose.
It is a 6C ketose sugar.
It is present in honey and fruits in the form of sucrose and inulin
Its phosphates are intermediates of glycolysis.
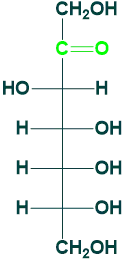
Sedoheptulose:
It is also known as cellulose
It is (7C) ketose sugar
It is found in plants
Its 7-phosphate is an intermediate in the hexose monophosphate shunt and photosynthesis.

Types of Monosaccharides Based on Number of Carbon Atoms:
Based on the number of the carbon atom, if an aldehyde group is present, then these are regarded as trioses (3C), tetroses (4C), pentoses (5C), hexoses (6C), heptoses (7C), octoses (8C), and nonoses (9C).
If a ketone group is present, then these are triulose (3C), tetrulose (4C), pentulose (5C), hexulose (6C), heptulose (7C),
Structure of Glucose and Fructose:
Glucose and fructose are monosaccharide sugars, and they are most important physiologically and medically. The structures of glucose and fructose can be represented in the following ways.
- The straight-chain structural formula (Fischer projection)
- Cyclic formula (Ring Structure or Haworth Projection)
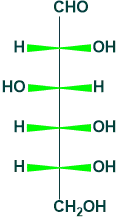
Fischer Projection of Glucose:
A 2D representation of the molecule is called a Fischer projection. The representation of glucose is the following.
Ring Structure or Haworth Projection:
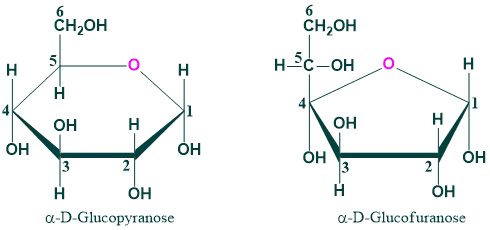
- Monosaccharide in solution is mainly present in ring form. In solution, a monosaccharide’s aldehyde (CHO) or ketone (C=O) group combines with its hydroxy (OH) group to generate hemiacetal or hemiketal respectively
- The alcohol (OH) group of C-5 and the aldehyde group of glucose at C-1 combine to produce the six-membered ring structure known as glucopyranose. Similarly, if the alcohol (OH) group at C-4 and the aldehyde group of glucose at C-1 react to create glucofuranose.
- However, in the case of glucose, the six-membered glucopyranose is much more stable than the furanose ring. In case of fructose the more stable form is fructofuranose.
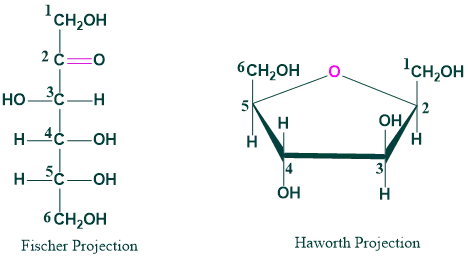
Fischer and Haworth Projection of Fructose:
Identification Test of Monosaccharides:
A chemical test known as the Barfoed’s test is used to identify the presence of monosaccharides and can identify reducing monosaccharides when disaccharides are present.
Oligosaccharides:
- Oligosaccharides consist of a short chain of monosaccharides(2-10 units).
- In oligosaccharides, monosaccharides held together by a bond are called glycosidic bonds.
- On hydrolysis of oligosaccharides, they break into 2 to 10 molecules of the simplest form of sugar called monosaccharides.
- They are also sweet.
- They are soluble in water
E.g. disaccharide, trisaccharide, tetrasaccharide, and pentasaccharide.
Disaccharides:
Among the oligosaccharides, disaccharides are the most common carbohydrates. It consists of two monosaccharide units. Two monosaccharides are held together by a glycosidic bond. Examples of disaccharides are maltose, sucrose, and lactose.
These are crystalline, water-soluble, and sweet. There are two types
- Reducing disaccharides
- Non reducing disaccharides
Reducing Disaccharides:
Reducing disaccharides with a free aldehyde or keto group e.g. maltose, lactose.
Maltose
Maltose is composed of two α-D-glucose units joined by glycosidic linkage between C-1 (anomeric carbon) of one glucose unit and c-4 of the other glucose unit.
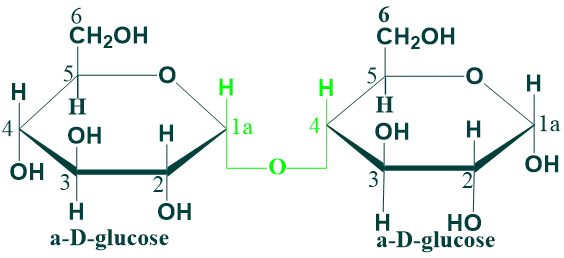
Maltose is a reducing disaccharide because free anomeric carbon of the second glucose unit which can act as a reducing agent. Maltose is produced as an intermediate product in the digestion of starch and glycogen by the action of the enzyme α-amylase.
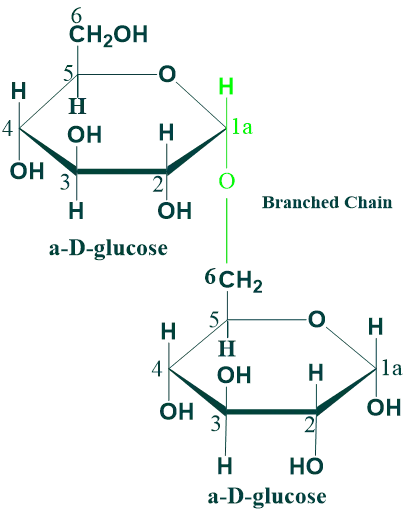
Isomaltose:
In isomaltose, the two glucose units are held together by α (1→4) glycosidic linkage. Isomaltose is a disaccharide derived from the digestion of starch or glycogen. It is hydrolyzed to glucose in the intestinal tract by the enzyme isomaltase.
Cellobiose:
Cellobiose is another disaccharide, identical in structure to maltose, except that the former has β (1→4) glycosidic linkage. Cellobiose is formed during the hydrolysis of cellulose.
Lactose:
Lactose is present in milk. Lactose contains one unit of β–galactose and one unit of β-glucose that are linked by a β(1→4) glycosidic linkage. The anomeric carbon of glucose unit is available for oxidation and thus lactose is a reducing disaccharide. Lactose is hydrolyzed to glucose and galactose by lactase enzyme in human beings. Lactose milk is the most important carbohydrate in the nutrition of young mammals.
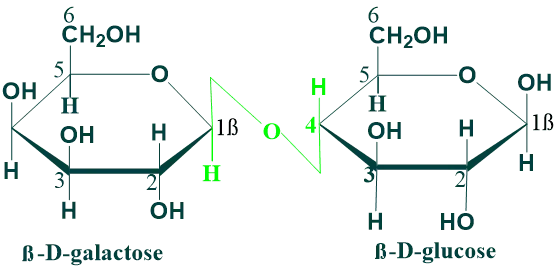
Non-Reducing Disaccharides:
Non-reducing disaccharides with no free aldehyde or keto group, e.g., sucrose.
Sucrose:
Sucrose is a disaccharide of glucose and fructose. It is formed by plants but not human beings. Sucrose is an intermediate product of photosynthesis. sucrose is also known as table sugar. The structure of sucrose consists of α-D-glucose and β-D-glucose. The two monosaccharides are held together by a glycosidic bond (α→β) between the C1 of the α glucose and the C2 of the β fructose.
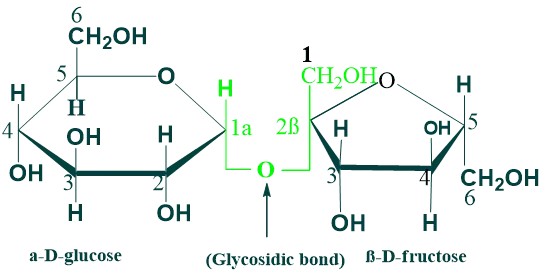
The reducing groups glucose and fructose are involved in glycosidic bonds; hence, sucrose is a non-reducing sugar, and it cannot form osazones. The intestinal enzyme sucrase hydrolyzes sucrose to glucose and fructose, which are absorbed.
Biomedical importance:
A variety of food preparations, including newborn and invalid foods, are made by hydrolysis of grains that contain high maltose content. From a dietary perspective. Consequently, they are simple to digest.
The lactose is synthesized in the lactating mammary gland for the baby, lactose from breast milk and glucose produced by the duct epithelium provide a good source of energy for newborns.
Typhoid bacillus, which is pathogenic (lactose nonfermenter), does not ferment lactose; instead, “Coliform” bacilli (E. coli), which are often non-pathogenic (lactose fermenter). The purpose of this test is to differentiate between these two microbes.
“Souring” of milk: Several microorganisms, including Str. lactis, A. aerogenes, and E. coli, convert the lactose in milk to lactic acid (LA), which is what causes the souring of milk.
Polysaccharides:
- Polysaccharides consist of a long chain of oligosaccharides (100 to 1000) or polymers of monosaccharides.
- They have high molecular weight.
- They are not sweet.
- Sparingly soluble in water.
- E.g., starch, glycogen, cellulose, and blood group polysaccharides.
Carbohydrate food list:
A variety of high-carb foods in our diets is key to fueling our bodies with energy and vitality. Here is the ultimate carbohydrates food list that encompasses fruits, vegetables, grains, and legumes
Fruits
Pineapples
Banana
Apple
Oranges
Berries
Mangoes
Vegetables
Beets
Sweet potatoes
Carrot
Corn
Peas
Grains
Buckwheat
Whole wheat Bread
Brown rice
Quinoa
Oats
Legumes
Blacked-eyed peas
Lentils
Black beans
Kidney beans
Split peas
Including these carbs foods in our meals and snacks can provide us sustainable energy throughout the day and contribute to our overall well-being
What are the benefits of carbohydrates?
- Sustain Energy and Improved Mood
They are broken down by the body into glucose which is utilized by our cells to produce energy. We remain active and awake because of this constant glucose flow, which also helps to prevent drowsiness. Furthermore, because they contribute to the synthesis of serotonin, a neurotransmitter connected to emotions of happiness and well-being, carbs have been linked to increased mood.
- Weight Management and Satiety
They can help with weight management. Whole grains, fruits, and vegetables are examples of high-fiber carbs that bring you a sensation of fullness and satiety, which can help you lose weight by preventing overeating. Carbs high in fiber also aid in blood sugar regulation, lowering the chance of insulin spikes that can cause cravings and weight gain.
- Heart Health and Cholesterol Management
Including healthy carbs in your diet, such as whole grains, fruits, and vegetables, can have a positive impact on heart health. Whole grains, in particular, contain soluble fiber that helps lower LDL cholesterol levels, often referred to as “bad” cholesterol. By incorporating these carbohydrates into your meals, you can support a healthy cardiovascular system and reduce the risk of heart disease.
- Digestive Health and Regularity
Fiber, a type of carbs found in plant-based foods, is crucial in maintaining a healthy digestive system. It adds bulk to our stool, preventing constipation and promoting regular bowel movements. Additionally, fiber acts as a prebiotic, providing nourishment for beneficial gut bacteria and promoting a balanced microbiome.