Wolff Rearrangement Definition:
When an α-diazo ketone is decomposed thermally, photochemically, or catalytically and converted to ketone by removal of dinitrogen, it is called Wolff rearrangement.
The reaction was given by Ludwing Wolf in 1902.
Reaction:
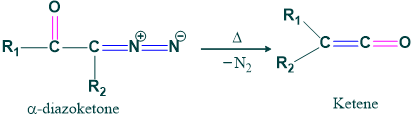
When ketene is formed, it acts as an intermediate for forming acid ester and amide.

Mechanism of Wolf Rearrangement:
Wolf rearrangement shows a concerted mechanism (single step) or also follows a stepwise mechanism, which is as follows:
Concerted Mechanism:
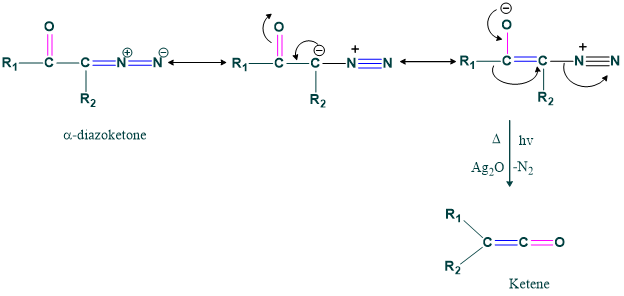
Stepwise Mechanism:
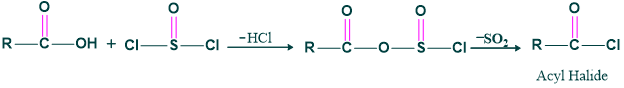
Step (1), Formation of acid halide:
Step (2), Formation of carbene:
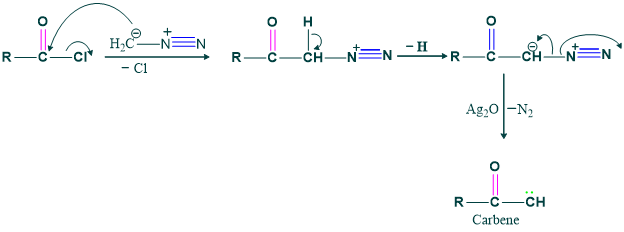
Step (3), Formation of ketene:
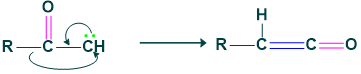
Application of Wolf Rearrangement:
The application of wolf rearrangement is as follows;
- Ring Contraction Reaction
- Higher Homologous
- Formation of oxygen (Photochemical Reaction)
(1) Ring Contraction Reaction:
Wolf rearrangement shows ring contraction reaction as follows:
Mechanism of ring contraction reaction:
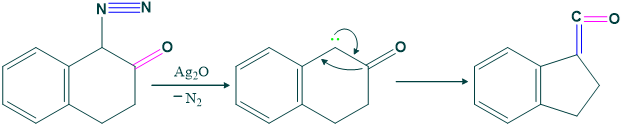
Higher Homologous:
By wolf rearrangement we increase carbon chain length.
Reaction :
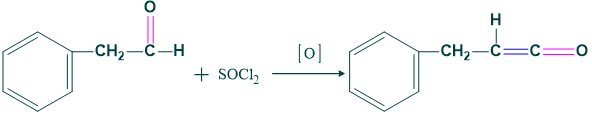
Here we use a selective oxidizing agent, which is pyridinium chlorochromate and manganese dioxide. Thus,
Mechanism:
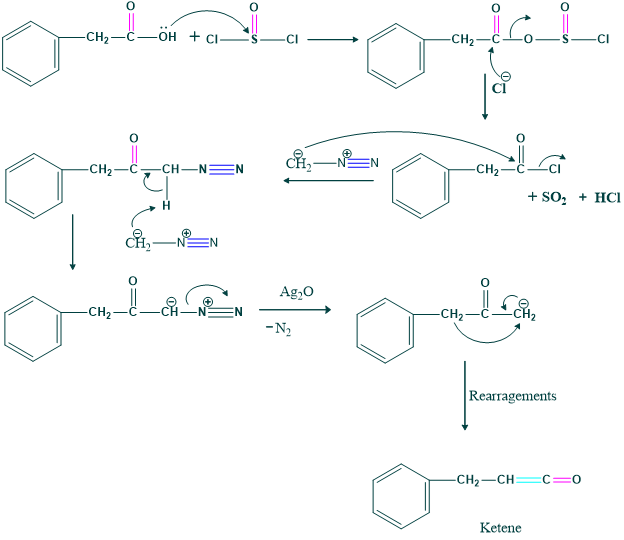
Formation of Oxyrene
It is also known as a photochemical reaction.
A 3-membered ring is relatively highly unstable and break to form carbene and then ketene.
Mechanism:
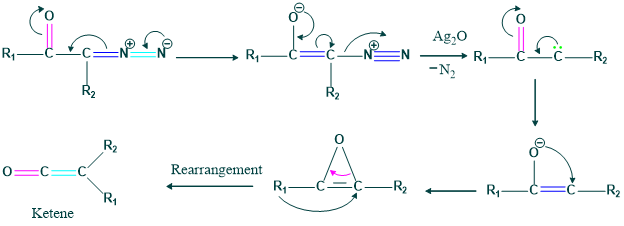
We also form carboxylic acid, ester and amine by different starting materials.