There are four different types of structures of proteins. The 20 amino acids commonly found in proteins are connected together by peptide bonds. The linear sequence of these amino acids encodes the information required to produce a protein molecule with a unique three-dimensional structure .
- Primary structure
- Secondary structure
- Tertiary structure
- Quaternary structure
Primary Structures of Proteins
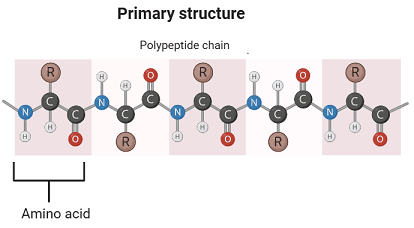
The arrangement of the amino acids that make up a protein’s backbone and the locations of any disulfide links are known as its primary structure.
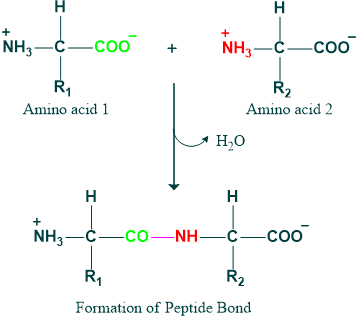
Formation of Peptide Bonds
In proteins, amino acids are covalently bonded by peptide bonds formed between the α-carboxyl group of one amino acid and the α-amino group of another, with the elimination of a water molecule.
Peptide Structures
The linkage of multiple amino acids via peptide bonds results in an unbranched chain called a polypeptide. Peptides containing more than ten amino acids (decapeptides) are referred to as polypeptides. Each amino acid in a polypeptide is termed a residue or moiety. Every polypeptide chain contains two free groups: the C-terminal, which is a free carboxyl group, and the N-terminal, which is a free amino group.
Characteristics of Peptide Bonds
The peptide bond is rigid and planar with partial double bond character. It generally exists in a trans configuration. The -C=O and -NH groups of peptide bonds are polar and participate in hydrogen bond formation. The peptide bond is uncharged at physiological pH; however, the peptides themselves carry a charge due to the terminal carboxyl and amino groups. Peptides are regarded as polyelectrolytes.
Importance of Peptide Bonds
Understanding the primary structure of a protein is crucial because many genetic diseases arise from an abnormal amino acid sequence. By knowing the primary structures of normal and mutated proteins, this information can be utilized for diagnosis or studying the disease.
Secondary structures of Proteins:
The regular folding and twisting of the polypeptide chain due to hydrogen bonding is referred to as the secondary structure of proteins. For the stability of the primary structure, hydrogen bonds form between the hydrogen of the NH and the oxygen of the C=O groups in the polypeptide chain, resulting in folding or twisting.
Two types of secondary structures are identified: α-helix and β-sheet.
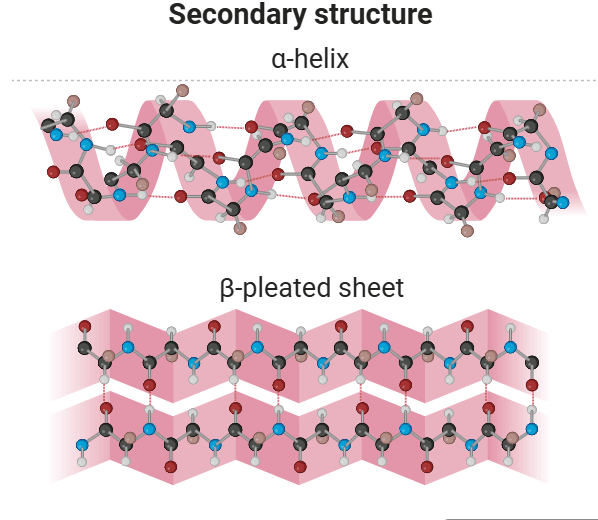
α-Helix:
The α-helix is a spiral structure of proteins characterized by a rigid arrangement of the polypeptide chain. Proposed by Pauling and Corey in 1951, this discovery marked a milestone in biochemistry. Key features of the α-helix include:
- A coiled, densely packed structure with side chains of amino acids extended from its center axis.
- Stabilization through extensive hydrogen bonding formed between the H atom attached to peptide N and the O atom attached to peptide C. These hydrogen bonds support the helix as a whole, even though they are weak individually.
- An optimal nitrogen-to-oxygen (N-O) distance of 2.8 Å for hydrogen bonding, with each amino acid’s CO group bonded to the NH of the amino acid four residues ahead in the sequence.
- An axial distance of 1.5 Å between adjacent amino acids results in 3.6 residues per turn of the helix.
β-Sheet:
Pauling and Corey also found another structure, which they termed the β-pleated sheet (β, because it was the second structure they identified). The β-sheet gets its name from the way its surfaces appear “pleated.”
- β-pleated sheets are made up of two or more completely expanded peptide chain segments, in contrast to the α-helix.
- In β-sheets, the hydrogen bonds are perpendicular to the polypeptide backbone rather than parallel, as observed in α-halix.
There are two possible ways that polypeptide chains can be arranged in β-pleated sheet conformation:
- Parallel pleated sheet
- Anti-parallel pleated sheet
Parallel Pleated Sheet
In a parallel pleated sheet:
- The polypeptide chains lie side-by-side and in the same direction (with respect to N- and C-terminals), so their N-terminal residues are aligned, stabilized by hydrogen bonds.
- Hydrogen bonds (interchain) form between the NH of one chain and the carbonyl (C=O) of a neighboring chain.
Anti-parallel Pleated Sheet
In the anti-parallel pleated sheet:
- The polypeptide chains lie in opposite directions, such that the N-terminal end of one chain is adjacent to the C-terminal end of the other.
- This structure is stabilized by interchain hydrogen bonding.
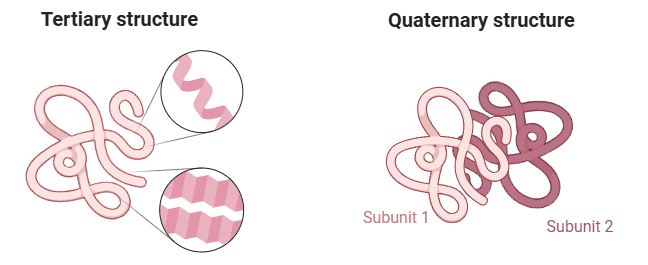
Tertiary Structure
The three-dimensional arrangement of a protein is referred to as its tertiary structure. This structure results from the further folding and twisting of the secondary structure about itself. Amino acid residues that are distant in the sequence can be brought close together by this folding, forming regions essential for the protein’s function, such as active or catalytic sites.
Tertiary Structure Stabilizing Forces:
A protein’s three-dimensional tertiary structure is maintained by:
- Hydrogen bonds
- Hydrophobic interactions
- Van der Waals forces
- Disulfide bonds
- Ionic (electrostatic) bonds or salt bridges.
Quaternary structures of proteins:
Quaternary structure is present in proteins with more than one polypeptide chain (polymeric), also known as oligomers. Single polypeptide chains are termed monomers or subunits. A dimer consists of two polypeptides, while a tetramer comprises four. The following are some instances of proteins with quaternary structures:
- Lactate dehydrogenase
- Pyruvate dehydrogenase
- Hemoglobin