What is the definition of molarity?
The definition of molarity is the number of moles of solute dissolved per dm³ of the solution.
OR
2. Another definition of molarity is the substance per unit volume of solution.
3. Molarity is also known as the
• amount concentration
• molar concentration
• substance concentration
o It is represented as M.
Formula of Molarity
The formula of molarity is given below:
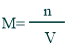
Calculating Molarity:
It can be calculated by the given equation; that is, the ratio of the moles of solute whose molarity is to be calculated and the volume of solvent is used to dissolve the given solute.

Unit of Molarity:
According to the International System of Units (SI), the unit of molarity is mol/m3.
• But the SI unit is not applicable for laboratory purposes, and most of the chemical literature uses the traditional unit that is mol/dm3 or mol/L.
• This traditional unit is mostly designated as the letter M.
• But in the SI prefix, “mega” is used for submultiples.
mol/m³ = 10⁻³ mol/dm³ = 10⁻³ mol/L = 10⁻³ M = 1 mmol/L = 1 mM
The units of millimolar and micromolar are designated as mM (10⁻³ mol/L) and µM (10⁻⁶ mol/L), respectively.
Frequently Asked Question definition of molarity
What is the relationship between molarity and molality?
Their relationship is mentioned below:
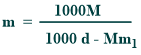
Where,
d=the density (g/ml)
m1=the molar mass of the solute
M = molarity
m=molality
What is the principle of molarity?
The main principle of molarity is that the mole of solute and volume of solution are used. The solute is that substance of a solution that is dissolved in the solvent component. A solvent is that component of a solution that dissolves the solute.
Does molarity depend upon the temperature?
Yes, molarity depends upon the temperature factor because volume of solution depends on temperature . As a result , molarity is also temperature dependent.
What increases molarity?
Molarity is inversely proportional to the volume of solution. So, if the volume of a solution increases, the molarity decreases and vice versa.
What is the difference between molarity and molality?
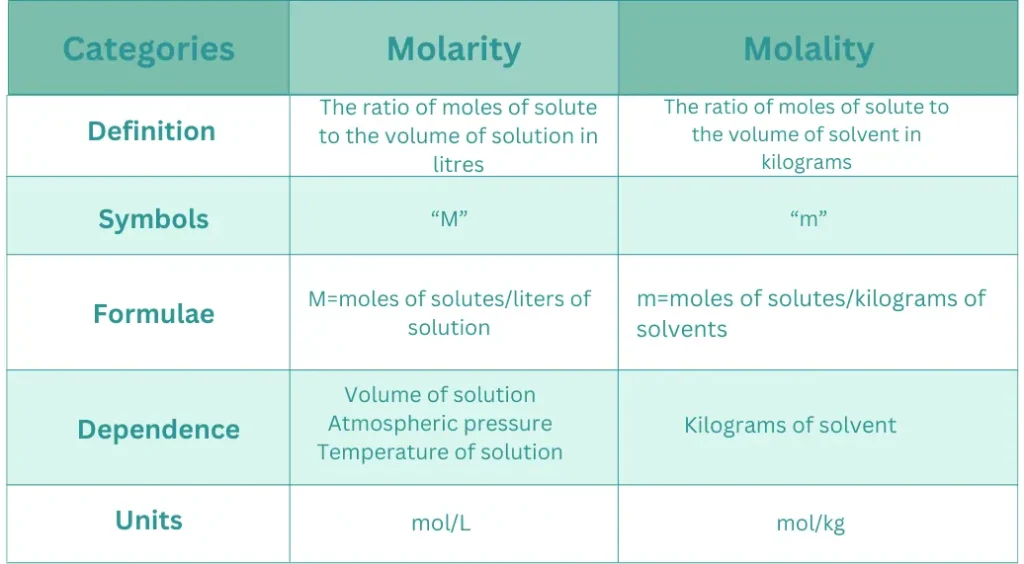
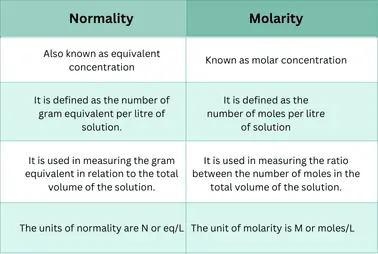
What is the difference between molarity and normality?