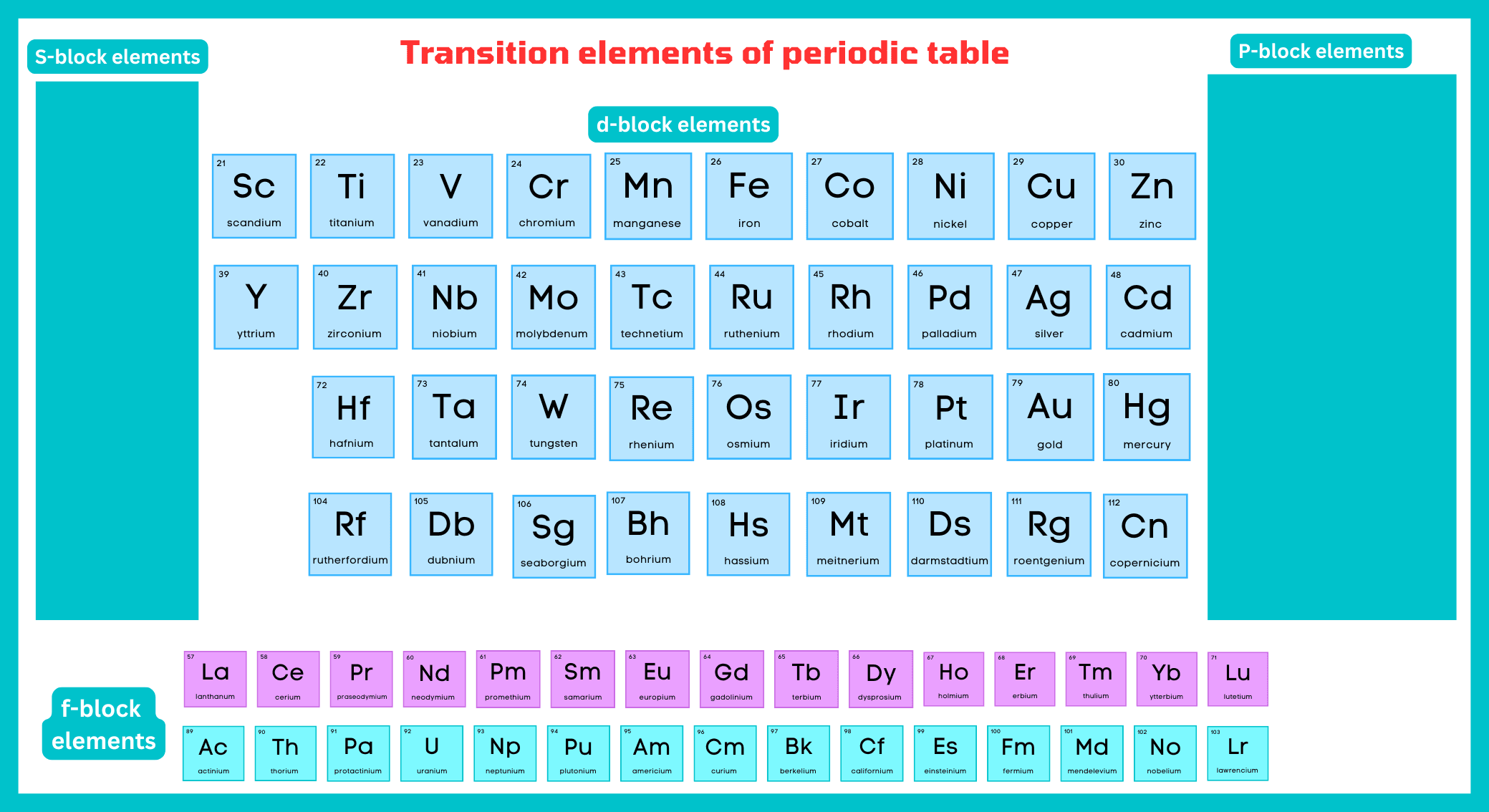
What are the transition elements?
[ez-toc]
Those elements, which have partially filled d or f-subshells in atomic state, are called transition elements. The d-block and s-block elements are transition elements because they are located between the s and p block elements.
These elements are divided into two types.
- Outer transition elements
- Inner transition elements
Outer Transition Elements:
Elements with partially filled d-subshells are referred to as d-block or outer transition elements. Cadmium, zinc, and mercury are also included in the transition elements, although they have no partially filled d-orbital. Copper in zero state has no d-subshell partially filled, but Cu+2 has configuration [Ar] 3s2, 3p6, 3d9, and transitional in character. The reason is that Zn, Cd, Hg, and their divalent cations should be excluded from the d-block element since they do not have partially filled (n-1) d orbitals. Similarly, Cu, Ag, and Au and their monovalent cations are also not considered to be transition metals or ions. In order to maintain a rational classification of elements, the elements are generally considered with d-block elements.
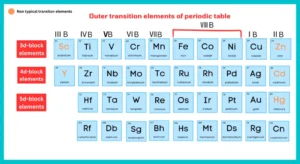
As we have discussed above (Zinc, Cd, Hg) of group ⅡB and (Sc, Ya, La) of group ⅢB are called non-typical transition elements.
Group ⅡB:
According to definition of transition elements, Zn, Cd, and Hg should not be placed with d-block elements because they do not have a partially filled orbital either as element or in any oxidation of their oxidation state.
Group ⅢB:
They have one electron in the d-subshell of their atoms. But in compounds, they mostly occur as the tripositive ions (Sc+3, Y+3, La+3) having no d electrons. in this way, they exhibit the properties of the main group elements.
Properties differing from transition elements of non-typical elements
- These elements (Zn, Cd, and Hg) are diamagnetic.
- They do not show a variable oxidation state.
- Most of their compounds are colorless.
General Properties of D-block Elements:
Oxidation state:
D-block elements exhibit variable oxidation state. They exhibit varied valencies due to the unpaired d-electrons’ participation in bond formation alongside the s-electrons.. These shifting of electrons is due to small energy differences between (n-1)d orbitals and ns orbitals of transition elements. All 3d series elements show an oxidation state of +2 in addition to higher oxidation states.
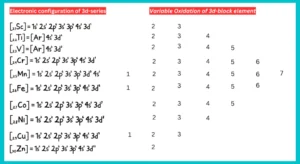
The +2 oxidation state is shown when only the 2s electrons are involved in bonding. In the highest oxidation state of the first five elements, all the s and d electrons are used for bonding. Manganese has the highest oxidation state (+7) among the the 3d-series transition elements. After Mn, the number of oxidation state decrease as the d-subshell fills up and fewer unpaired electrons are available for bond formation.
Electronic Configuration of 4 and 5d-series elements:

Paramagnetism:
Those substances that are weakly attracted by a strong magnetic field are called paramagnetic substances. e.g.MnSO4, FeCl2, NiCl2
Diamagnetic compounds are those that are just slightly repelled by strong magnetic fields, e.g., ZnCl2, ScCl2, CuCl, etc
Reason of Paramagnetism:
Paramagnetism behaviour is caused by the presence of unpaired electrons in an atom , molecule or ion because there is magnetic movements associated with spinning of electrons. It rises as the number of unpaired electrons rises.
When electrons are paired in an orbit, then magnetic movements are cancelled out and the substances become diamagentic.
Melting and boiling points:
The transition metals have very high melting and boiling as compared to those of s-block elements
Metallic Character:
All the d-block elements are metals since the number of electrons in the outermost shell is very small, being equal to 2. They are ductile, flexible, and hard. These metals are effective heat and electrical conductors.
Alloy Formation:
Along with other elements, they also combine to form alloys. Alloy steel consists of chromium, manganese, and nickle atoms.
Brass, bronze, German silver, and gum metal are also examples of alloys.
Inner Transition Elements:
The elements having f-subshell partially filled in their common oxidation are called f-block elements. Inner transition elements are another name for f-block elements.
f-block elements include two rows at the bottom of the periodic table. The first row is called lanthanides, while the second row is called actinides. All actinides are radioactive in nature.
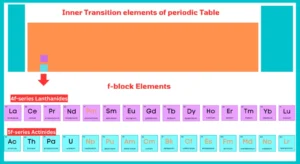
Lanthanides:
The fifteen elements from lanthanum (At. n0.57) to lutetium (At. no. 71) are known as lanthanides or rare earth because they were obtained as earth (oxides) from relatively rare minerals.
Name of Lanthanides:
Lanthanum, Cerium, Neodymium, Promethium, Samarium, Europium, Gadolinium, Terbium, Dysprosium, Holmium, Erbium, Thulium, Ytterbium, and Lutetium
Properties of Lanthanides:
Electronic Configuration:
The general electronic configuration of the element is
![]()
Lanthanum electronic configuration and lutetium electronic configuration have no partially 4 f orbitals in their ground state and are considered lanthanides due to their properties to these elements.
![]()
Oxidation state:
Although +3 is the typical oxidation state of lanthanides, many elements can also have +2 or +4 oxidation states, which result in the stable ions they leave behind .eg.
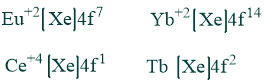
Lanthanides Contraction:
As the atomic number of the lanthanide elements expands, the radii gradually reduce. An electron moves to the subshell for each new proton delivered to the nucleus. The f-orbitals have a weak shielding effect and an extremely jumbled form. The electron charge cloud’s size contracts as a result of an increase in effective nuclear charge. This steady contraction of size is called a lanthanide contraction.
Consequences of Lanthanides Contraction:
- Covalent character increases of the cations.
- The electronegativity of trivalent ions slightly increased.
Electronegativity:
Electronegativity values of lanthanides are almost the same as those of those s-block elements. Lanthanides form ionic compounds.
Reactivity:
Due to their low value of ionisation energies, the lanthanides are very reactive.
Alloy Formation:
They form alloys, particularly with iron, e.g., MISCH METAL, rare earth.
Actinides:
The fifteen elements from actinium (At. no. 89) to lawrencium (At. no. 103) are known as actinides and constitute the 5-f series. From neptunium to onwards the elements are man made ( artificially prepared) and are known as transuranium elements.
Name of Actinides:
Actinium, Thorium, Uranium, Neptunium, Plutonium, Americium, Curium, Berkelium, Californium, Einsteinium, Fermium, Mendelevium, Nobelium, and Lawrencium.
Properties of Actinides:
Electronic Configuration:
The differentiating electron enters the 5f atomic orbitals. Their general electronic configuration is
![]()
Oxidation State of Actinides:
The common oxidation state is +3 but other oxidation state are also exhibited by actinides, with the maximum being+7
Actinides Contraction :
It is similar to lanthanide contraction due to poor shielding of electrons.
Complex Formation:
They have higher tenancy to form complex compounds.