The reaction in which aldehyde or ketone react with phosphorus ylide to give alkenes and triphenyl phosphine oxide. Actually ylide is a neutral molecule having a negative carbon adjacent to a positive heteroatoms. This phosphorus ylide is also called phosporanes. Thus phosphorus ylide is easily prepared from triphenyl phosphine and primary or secondary alkyl halides. This reaction is as follows;

Witting Reaction Mechanism:
Step(01):
In this step, ylide or triphenyl phosphine is a good nucleophile and attacks on aldehyde or ketone to give betaine, which may or may not formed.
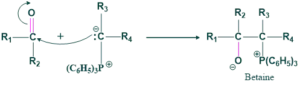
Step(02):
In this step, betaine is converted to oxaphosphetane ( cyclic intermediate).

Step(03):
In this step, breaking of cyclic intermediate( oxaphosphetane) takes place to give alkene and triphenyl phosphine oxide.
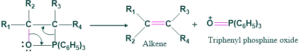
Wittig Reaction Ylide Preparation:
Witting reaction ylide preparation takes place in two steps.
Step(01):
In this step nucleophilic substitution reaction takes place. Triphenyl phosphine is a good nucleophile and a weak base. Thus primary or secondary alkyl halide react with triphenyl phosphine. This takes place by SN2 mechanism. Thus triphenyl phosphine displaces halide ion from alkyl halide to give alkyl triphenyl phosphonium salt.

Step(02):
The second step shows acid-base reaction. A strong base like alky lithium (R-Li) or phenyl lithium ( C6H5Li) removes a proton from the carbon gives ylide. Phosphorus ylide can be represented as hybrid of resonance structure.
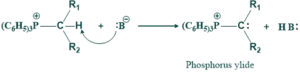
Two resonance form of ylide
