UV-Vis spectroscopy
UV-Vis spectroscopy is also known as electronic spectroscopy, which involves the absorption of electromagnetic radiation and results in the excitation of electrons from low-energy molecular orbitals to high-energy molecular orbitals. The UV-Vis region absorbs radiation in the range of 200-400 nm. This type of spectroscopy is used to find aromatic conjugation within molecules.
♦What is Spectroscopy?
Spectroscopy is defined as a “measure to study the number of electromagnetic radiations absorbed or emitted by a substance”.
OR
“Study of interaction of electromagnetic radiations with the matter”.
The result of absorption of electromagnetic radiation by a substance is recorded as a plot of absorbance versus wavelength known as absorption on the spectrum.
♦What are the types of electronic transitions in UV spectroscopy?
By the absorption of electromagnetic radiations, the transition of valence electrons from occupied molecular orbital like a nonbonding n or bonding σ or π to next high energy orbitals like antibonding σ* or π* occurs. These electronic transitions are of four types;
Figure no 1 types of Electronic Transitions
1) σ – σ* transitions:
This type of transition occurs in saturated hydrocarbons having σ electrons and because the sigma bond is highly strong it requires a high amount of energy for transition from ground state to excited state, generally at wavelength below 150nm.
⇒ For example;
In the case of alkanes, a C-C bond absorbs at 135nm, and a C-H bond absorbs at 125nm.
2) n-σ* transitions:
This type of transition occurs in saturated hydrocarbons containing a heteroatom(having a lone pair of electrons) like oxygen, sulfur, halogens, etc. This transition requires comparatively less amount of energy as compared to σ – σ* transitions.
⇒ Examples ;
Water absorbs at 167nm; methanol absorbs at 174nm; methyl chloride at 169nm; methyl iodide at 258nm.
◊ Why methyl iodide absorbs at a higher wavelength than methyl chloride?
In the case of saturated alkyl halides; as the size of halogens increases, electronegativity decreases, so the electron from large-sized halogen requires less amount of energy for transition to an excited state generally at a higher wavelength. That’s why methyl iodide in which iodide is larger absorbs at a higher wavelength as compared to methyl iodide for electronic transition.
3) π-π* transitions:
This type of transition occurs in unsaturated hydrocarbons containing one or multiple bonds and requires less amount of energy even than n-σ*.
⇒ Examples;
In simple alkenes, the absorption band occurs at 170-190nm.
◊ How does conjugation affect wavelength in UV Vis spectroscopy?
As conjugation increases, the no. of p orbitals increases, as a result, the energy gap between HOMO-LUMO decreases, and the π-π* absorption shifts towards a longer wavelength. For example; ethylene CH2=CH2(no conjugation) absorbs at 171nm; whereas 1,3-Butadiene(having 2 conjugated double bonds) absorbs at 217nm.
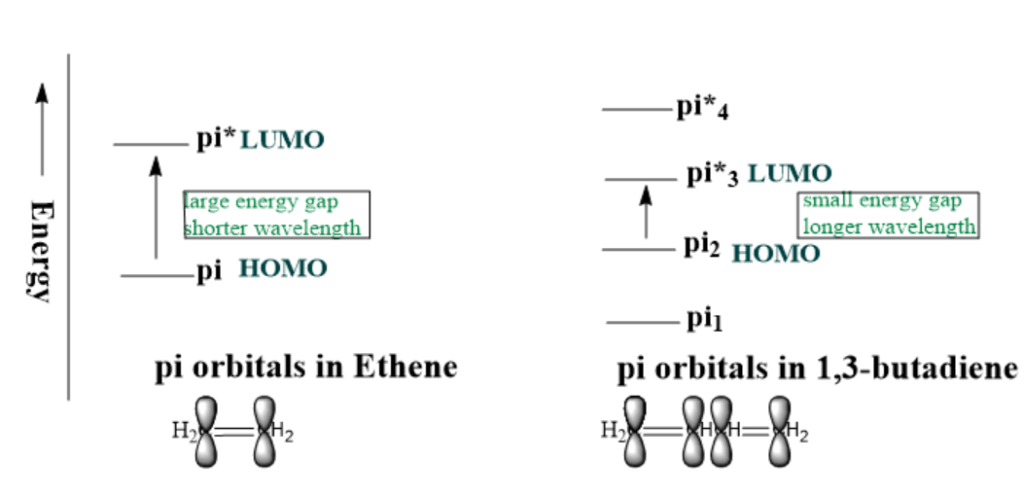
Figure no: 2 Effect of conjugation on wavelength in case of ethene and 1,3 butadiene
4) n-π* transitions:
This type of transition occurs in unsaturated compounds containing a heteroatom( having a lone pair of electrons). These transitions require the least amount of energy for the electronic transition from nonbonding molecular orbital to antibonding molecular orbital π*.
⇒ Examples;
Saturated aldehydes and ketones absorb at 275-295nm.