What is tautomerism?
- Tautomerism is a phenomenon in which a hydrogen atom’s movement and a double bond’s shifting take place.
- It refers to isomers that can easily interconvert.
- A chemical phenomenon in which a single chemical molecule exists in two or more interconvertible forms with different placements of a proton and double bond. These various structures are known as tautomers, and such processes are known as tautomerism in chemistry. Interconversion between these structures is often quick and occurs in a dynamic equilibrium condition.
- The most prevalent type of tautomerism is keto enol tautomerism, which occurs when a molecule with a keto group (C=O) is transformed into its enol counterpart (C=C-OH). For example, in acetoacetic acid, the keto form is CH3-CO-CH2-COOH, while the enol form is CH3-C(OH)=CH-COOH.
- It is a structural isomerism significant in many chemical and biological processes.
- Tautomers and contributing structures in chemical resonance are two different terms; don’t confuse them.
Examples:
Enol-keto form:

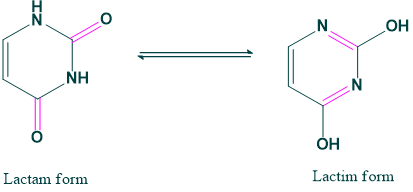
Lactam-Lactim form:
Amide-Imidic acid form:

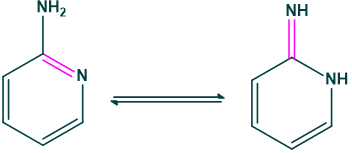
Amine-Imine form:
Conditions for tautomerism:
- That compound must contain an electron-withdrawing group that can abstract the hydrogen atom.
- The carbonyl group must contain an alpha hydrogen atom.
- keto enol tautomerism type proceeds in the availability of acidic or basic catalyst.
Structural requirement of tautomerism in chemistry:
- Those compounds contain polar molecules and weakly acidic functional groups.
- Involvement of change in the position of an atom.
- It does not affect the bond length or such features.
- Normally, it occurs in planar or non-planar molecules.
Types of tautomerism:
1. Prototrophy:
The process that arises due to the transfer of a proton within a molecule, i.e., amino acid, which is the building block of protein, is called prototrophy.

2. Annular tautomerism:
The process that arises due to the shifting in a heterocyclic system.

3. Ring chain tautomerism:
The phenomenon that arises due to the shifting of protons from the open chain and converting them into a closed chain.
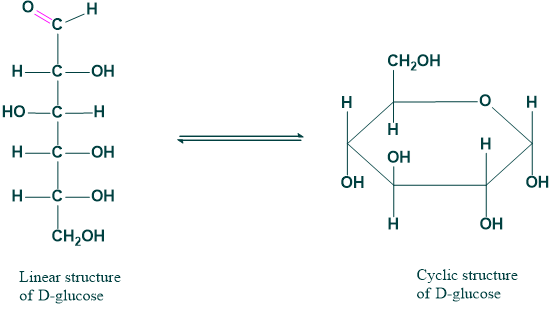
4. Non-carbonyl tautomerism:
The phenomenon that arises due to the shifting of protons in noncarbonyl compounds.



5. Valence tautomerism:
That phenomenon arises due to the continuous formation and breaking of single and double bonds in the compound without any migration of groups or atoms.
The molecular geometry of a molecule is changed in valence tautomerism and is not confused with canonical resonance structures.
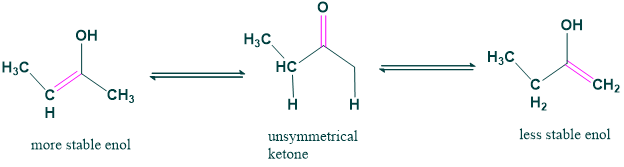
6. Tautomeric form of unsymmetrical ketones:
There is only one form of tautomer that occurs in symmetric form. However, for an unsymmetrical form, there can be two tautomeric forms.
7. Keto enol tautomerism:
- The process that arises due to the conversion of keto form into enol form by the action of a small amount of acid or base as a catalyst is called keto-enol tautomerism.
- Tautomers are constitutional isomers that are readily interconvertible. These are differentiated based on the placement of a labile hydrogen atom and the position of the double bond.
- The equilibrium between tautomers is not only rapid under normal conditions but often strongly favors one of the isomers. For example, Acetone is 99.999% keto tautomer and ~0.001% enol tautomer.
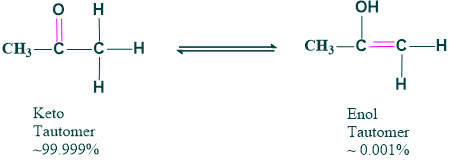
- The minor tautomer and the major tautomer are formed due to the chemical behavior of the compound.
- Traces of acids or bases are used as catalysts for the tautomeric equilibrium.
- A keto tautomer favors the rapid equilibrium than the enol tautomer. But in some other reactions, enol is the most important tautomer.
Case 1: Intramolecular H-bonding
One example is the 1,3-diketones, such as acetylacetone (2,4-pentanedione), which prefer the enolic form. Intramolecular H-bonding creates a 6-membered cyclic structure that stabilizes the enol form over the keto.
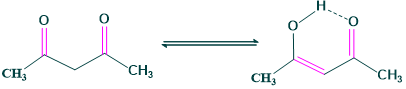
Case 2: Aromaticity
In some aromatic compounds, i.e. phenol, the enol exhibits the aromatic character so it is more favorable than ketone.
Case 3: Solvent
Polar solvents ( which can form hydrogen bonding ) will make lone pairs less available for intramolecular H-bonding, hence % of keto increases. aromatic character
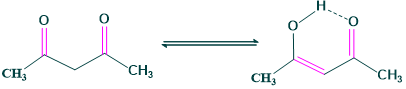
keto: enol
6: 94 (when benzene is used as solvent)
81: 19 ( when water is used as solvent)
Case 4: Conjugation
conjugated enol is more favored for ketone than non-conjugated enol.
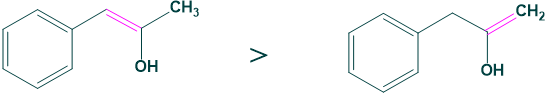
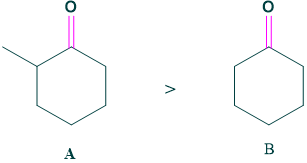
Case 5: Substitution
more substituted ketone (A) is more favored for enol than (B) ketone.
Recommended videos:
part -1 :
Part -2 :