Chemical Reactions of Monosaccharides
The most important chemical reactions of monosaccharides are:
- Action of strong acids: Furfural formation
- Enolization or Tautomerization
- Oxidation or Sugar acid formation
- Reduction or Sugar alcohol formation
- Osazone formation or Action of phenylhydrazine
Furfural formation:
Sugar loses water when heated with acid substances (H₂SO₄ or HCl) and generates furfural derivatives. It is also known as the dehydration of monosaccharides. Thus, hexose gives hydroxymethyl furfural, while pentose gives furfural on dehydration. This furfural can condense with phenolic compounds (α-naphthol) to form colored products. This is the chemical basis of the popular Molisch test.
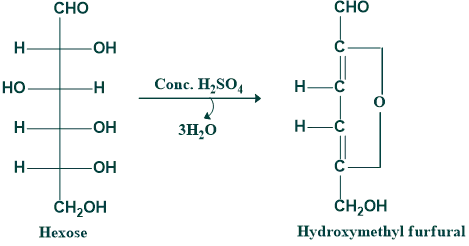
In the case of oligo- and polysaccharides, they are first hydrolyzed to monosaccharides by acid, and then the dehydration step will occur.
Bial’s Test:
Pentose reacts with strong HCl to form furfural derivatives and then reacts with orcinol to form a green-colored complex. Bial’s test is used for detection of xylose in urine and helps to identify pentoses by producing a characteristic bluish-green color, distinguishing them from hexoses.
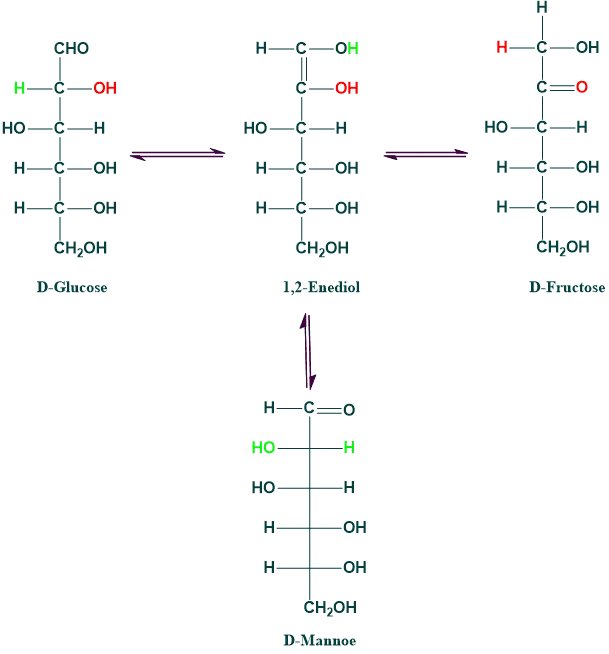
Enolization:
The process of shifting a hydrogen atom from one carbon atom to another to produce enediols is known as tautomerization or enolization.
OR
Applying diluted aqueous alkalies causes aldoses and ketoses to convert into enediols. Enediol is the enol form of sugar because two OH groups are attached to the double-bond carbon.
- Enediols are good reducing agents and form the basis of Benedict’s test and Fehling’s test.
- Through the formation of common 1,2-enediol, glucose, fructose, and mannose may isomerize into each other in a dilute alkaline solution.
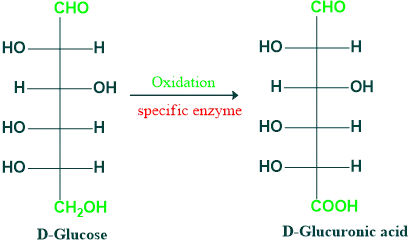
Oxidation Reactions of Monosaccharides:
When aldose oxidize under proper conditions, they may form a
-Aldonic acid
-Saccharic acid
-Uronic acid
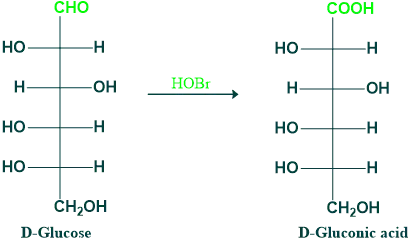
- Oxidation of aldose with hypobromous acid (HOBr), which acts as an oxidizing agent, gives aldonic acid, e.g., oxidation of D-glucose into d-gluconic acid
When aldoses are oxidized with nitric acid in the right circumstances, both aldehyde and terminal primary alcohol groups are converted to carboxyl groups, which results in the formation of saccharic acid.
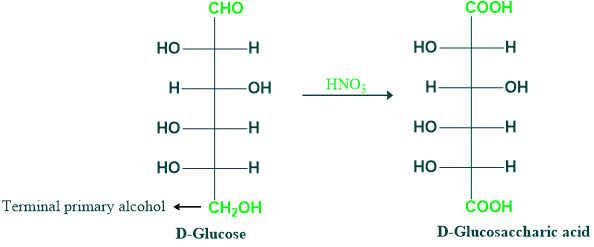
HNO₃ is a strong oxidizing agent as compared to HOBr, so this is the reason HNO₃ oxidizes the aldehyde and terminal primary alcohol group, and the resulting dicarboxylic acid that is formed is called aldaric acid (old terms are saccharic acid or glycaric acid).
- Uronic acid is created when an aldose is oxidized in a way that converts the terminal primary alcohol group to carboxyl without oxidizing the aldehyde group (often through the use of particular enzymes).
Reduction of Monosaccharides:
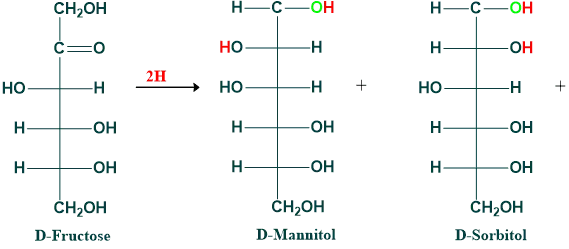
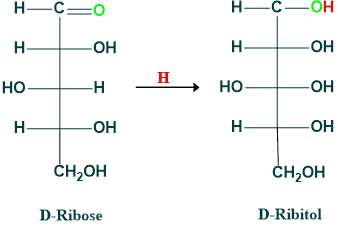
Alcohol is produced when monosaccharides are reduced. Alcohols formed from glucose, mannose, fructose, galactose, and ribose are given below.
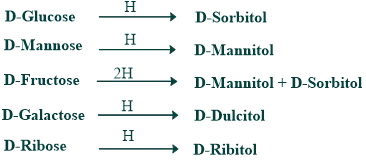
What is the reduction reaction of glucose to form sorbitol?
When glucose is reduced, the aldehyde functional groups in the glucose change into the alcohol group, which is known as sorbitol.

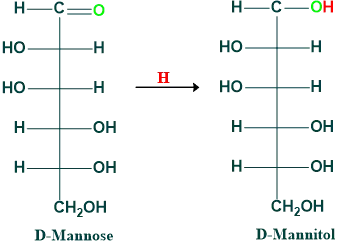
Reduction of Mannose:
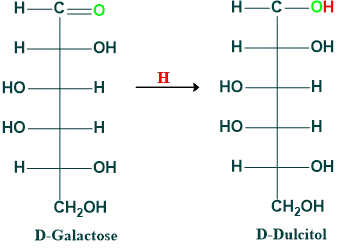
Reduction of Galactose:
Reduction of Fructose:
Reduction of Ribose:
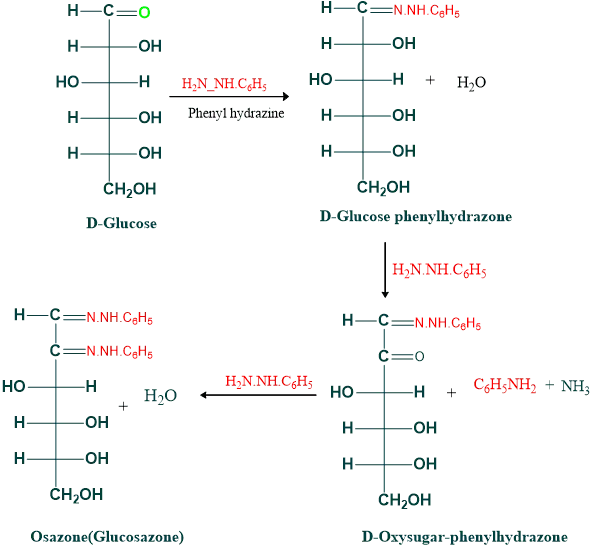
What is osazone formation?
- Osazones are crystalline, yellow, or orange products obtained by reducing sugar with phenylhydrazine.
- Due to their similarity in the lower four carbon atoms, glucose, mannose, and fructose produce identical osazones.
- The first two carbons (C1 and C2) are involved in osazone formation
The reaction of glucose with phenylhydrazine is given below.
The shapes of the reducing disaccharides, lactose, and maltose, and the osazone crystals of glucose vary
– Glucosazone is needle-shaped:
– Lactosazone is formed like a powder puff or tennis ball;
– Maltosazone is fashioned like a sunflower.