Qualitative test for Carbohydrates
The qualitative tests for carbohydrates such as (Molish, Benedict, Fehling, Osazone, and Barfoed tests) are used to detect the presence of reducing and non-reducing, mono and disaccharides in a sample. Here are common qualitative tests for carbohydrates:

EXPERIMENTAL ANALYSIS
1. Molish Test
The molish test is a simple, rapid, and chemical-sensitive test to detect the presence of carbohydrates (sugars, and starches) in a sample.
Principle
Dehydration of carbohydrates from the purple compound with α-naphthol.
Requirements
- α-naphthol
- conc. sulfuric acid
- sample solution
- conical flask
- tripod stand
- iron stand
- beakers
- stirrer
- test tubes
- thermometer
Procedure
- Prepare a sample solution
- Add 2 to 3 drops of α-naphthol to the sample
- Carefully add 1 to 2 ml of con. sulfuric acid
- Mix it well
- Observe the purple ring or violet color.
Results
- The purple color indicates a positive test
- no color change means a negative test (no carbohydrates)
Application
- clinical diagnosis (urine glucose testing)
- food analysis (presence of sugar)
- different biochemical research works
Limitations
- The non-specific test detects all types of carbohydrates.
- False positive with non-carbohydrate compounds.
2. Fehling test
Fehling test is a chemical test that is used to detect the presence of reducing sugars such as monosaccharides and disaccharides in a sample.
Principle
Reducing sugars reduces copper || ions to copper| ions, forming red precipitates.
Requirements
- Fehling solution A copper|| sulphate
- Fehling solution B sodium hydroxide and Rochelle salt
- sample solution
- titrating apparatus
- water bath
Procedure
- Make a sample solution in a clean and dry beaker.
- Mix 01ml of Fehling A and 01 ml of fehling B, stirr it well.
- Add 1 to 2 ml of sample.
- Boil the solution for 05 to 10 minutes.
- Observe for red precipitates.
Result
- red precipitates for a positive test
- no precipitates mean a negative test
3. Benedict test
Benedict test is a test that indicates the strength of reducing sugars such as strong, weak, and moderate.
Principle
Reducing sugars reduces copper|| to copper| ions that form a colored compound.
Requirements
- Benedict reagent
- sample
- titrating instruments
- boiling apparatus
Procedure
- Make a sample solution in water
- mix 1ml of benedict reagent with 10ml of sample.
- boil it for 05 minutes.
- observe for color changes.
Results
- Blue color for negative test
- green for reducing sugars
- yellow for moderate reducing sugars
- orange red shows the presence of strong reducing sugars
4. Barfoed test
Barfoed test is a chemical test used to detect the presence of monosaccharides and disaccharides ( classification of lipids). It’s a useful test in biochemistry and food science. Here’s how it works:
Principle of Barfoed test
- Reducing sugar such as mono and disaccharides reduced copper acetate present in the reagent into copper oxide in an acidic medium.
- sugars with hemiacetal are in equilibrium with an open chain form in a solution and behave as a reducing agent towards containing metal salts
- This test relies on the ability of monosaccharides to reduce copper ll ions to copper l ions, forming a red precipitate of copper l oxide, disaccharides, and polysaccharides do not react with the reagent under these conditions.
Barfoed Reagent
- 0.33M copper acetate in dist. water
- Acetic Acid ( 1% solution )
- Dissolve 1.1g of copper acetate in 20ml distilled water and filter out the solution
- Add 0.18ml acetic acid infiltrate
- Copper acetate with acetic acid decreases the Ph and makes the medium acidic
Apparatus
- Beakers
- stirrer
- thermometer
- tripod stand
- conical flask
- water bath
- heating apparatus like spirit lamp
Procedure
- Take three separate test tubes, clean them, and label as monosaccharides, disaccharides, and control respectively.
- Add 1ml sample in both mono and di saccharides labeled tubes
- Add 1ml sample dist. water in the control tube.
- Add 3 ml of Barfoed reagent in all the test tubes.
- Place the tubes in a water bath.
- Observe the color change.
Limitations
- The test is not specific to a particular monosaccharide.
- Some disaccharides may give a false positive result if they are partially hydrolyzed to monosaccharides during the tests.
Result of this qualitative test
- Glucose (mono) gives precipitates of brick reddish brown color in 2- 3 mins.
- Disaccharides (maltose) give ppt in 10 mins.
- The control tube remains unchanged, which means the absence of monosaccharides (glucose, fructose) and the presence of disaccharides (e.g. sucrose) or polysaccharides.
The Barfoed test is a simple and useful tool for detecting monosaccharides, but it should be used in conjunction with other tests for more specific identification.
5. Salliwanoff’s test
Salliwanoffs test is a chemical qualitative test used to detect the presence of cholesterol, aldose, and ketose sugar in a sample. This test is simple, rapid, and sensitive, and should be used with other tests for confirmation.
Applications
- Food analysis( e.g. cholesterol in oils)
- Pharmaceutical analysis
- Clinical diagnosis (e.g. cholesterol levels in blood)
Principle
A dehydration reaction due to hydrolyzed groups of sugar. Salliwanoffs reagent is resorcinol in dilute hydrochloric acid. Ketones ( e.g. fructose) are more readily dehydrated by HCl than the aldose to form hydroxyl methyl furfural which then condenses with resorcinol to form a red complex.
Requirements
- The apparatus is the same as we use in the Barfoed test
- Salliwanoffs reagent
- sample
- Salliwanoffs Reagent
- Dissolve 0.01gresorcinol in 6.6ml conc. HCl.
- Add distilled water up to 13.4ml
Procedure
- Take a test tube, and wash it with clean water.
- Add 01 ml sample (fructose) solution in it.
- Now, add 2ml reagents and mix them.
- Place the test tube in a boiling water bath.
- Focus on color changing.
Result
A reddish color appears in 1min if keto sugar is present. Faint pink appears for aldose sugar.
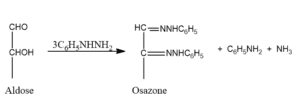
6. Osazone test
The osazone test is a chemical qualitative test used to detect and identify reducing sugars (monosaccharides and disaccharides)in a sample.
What is Osazone?
Carbohydrates derivative which is formed when any carbohydrate reacts with phenyl hydrazine to form osazone.
- Osazone is also known as 1,2- phenylhydrazine.
- Osazone is a different shape crystal for every monosaccharide.
- The differentiation is based on time for different reducing sugars.
Crystal color is because of phenylhydrazine.
Osazone reaction
Osazone Composition
- 2g phenyl hydrazine HCl+ 2g sodium acetate
- 9 -10 drops of glacial acetic acid
Procedure
- Take a test tube and add a 5ml sample into it.
- Now add freshly prepared phenylhydrazine mixture and boil it.
- Heat the mixture gently to 60- 80°C for 5- 10 minutes.
- Then, the formation of crystals.
- Observe the crystals under a microscope.
Result
- Positive result: Yellow, orange, or crystalline precipitate forms.
- Negative test: No precipitate forms.
Uses of Osazone test
It is used for the identification of reducing sugar
- Clinical diagnoses (urine glucose testing)
- food analysis( sugar detection)
- Biochemical research
Limitations
- Do not distinguish between specific reducing sugars.
- False positives with non-carbohydrate-reducing sugar.
read more
qualitative tests for carbohydrates