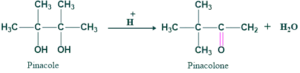
An Acid catalyzed rearrangement in which 1,2-diol or pinacole is converted to carbonyl compound is called pinacole- pinacolone rearrangement.
Pinacole : 2,3- dimethyl butane-2,3-diol
Pinacolone :3,3-dimethyl-2- butanone
Reaction of Pinacole-pinacolone rearrangement:
Mechanism of pinacole-pinacolone rearrangement:
Mechanism of pinacole-pinacolone rearrangement takes place in four steps
Step(01):
In this step hydroxyl group of pinacole is protonated by acid because reaction takes place in acidic medium.
Step(o2):
In this step water is removed and forming a tertiary carbocation, there this carbocation is stable .

Step(03):
In this step, methyl shift takes place to positively charged carbon ion.

Step(04):
In this step oxygen atom is deprotonated giving our product i.e. pinacolone.

Unsymmetrical Pinacolone:
In unsymmetrical pinacolone reaction we form a stable carbocation and we show methyl or either phenyl shift. Thus it show migratory aptitude. Migratory aptitude order is
![]()
Here we form
- Stable carbocation
- Migration of suitable group
- Regioselectivity
Reaction:
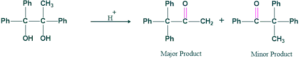
Mechanism Of Unsymmetrical pinacolone:
In Unsymmetrical pinacolone two possibilities occurs
First Possibilty:
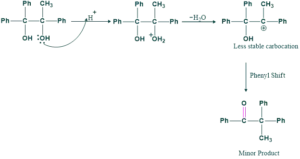
Second Possibility:

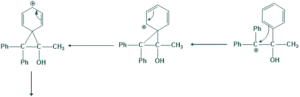
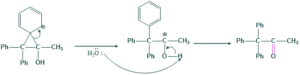
- More stable carbocation give more major product
- Less stable carbocation give minor product
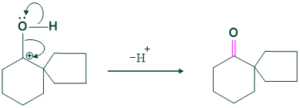
Cyclic Pinacole-pinacolone rearrangement:
Cyclic pinacole-pinacolone rearrangement forms spirocylic ring systems. This is also called ring expansion rearrangement.
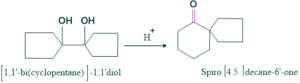
Mechanism:
Step(01)Protonation:
In this step protonation of hydroxyl group takes place in acidic medium as follows;
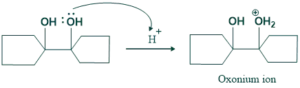
Step(02)Deprotonation:
In this step removal of water takes place for the formation of carbocation.

Step(03)Methyl shift:
In this step methyl shift takes place for the formation of stable carbocation.
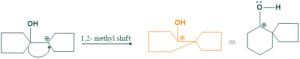
Step(04):
In this step deprotonation of oxygen takes place forming C=O group.