What is hemoglobin?
Hemoglobins are iron-containing proteins in the blood of many animals in the red blood cells (erythrocytes) of vertebrates that transports oxygen to the tissues. Haemoglobin forms an unstable, reversible bond with oxygen. In the oxygenated state, it is called oxyhaemoglobin and is bright red; in the reduced state, it is purplish blue.
Heme is a prosthetic group found in conjugated complex proteins that make up hemoglobin. The erythrocytes hemoglobin content is what gives blood its red hue.
Hemoglobins level:
Males typically have blood hemoglobin concentrations of 14–16 g/dl, whereas females typically have 13–15 g/dl. Two significant biological processes related to breathing are demonstrated by hemoglobin.
Males typically have blood hemoglobin concentrations of 14–16 g/dl, whereas females typically have 13–15 g/dl. Two significant biological processes related to breathing are demonstrated by hemoglobin.
Normal haemoglobins levels by age:
- Newborn: 14–24 grams per deciliter (g/dL)
- 0–2 weeks: 12–20 g/dL
- 2–6 months: 10–17 g/dL
- 6 months–1 year: 9.5–14 g/dL
- 1–6 years: 9.5–14 g/dL
- 6–18 years: 10–15.5 g/dL
- Adult men: 14–18 g/dL
- Adult women: 12–16 g/dL
- Pregnant people: >11 g/dL
Hemoglobin levels may decrease slightly in older adults. The prevalence of anemia increases with age, and some serious underlying conditions may lead to anemia in older people.
Structure of Haemoglobins:
Hemoglobin is made up of heme and globin parts. Heme + globin = Hemoglobin
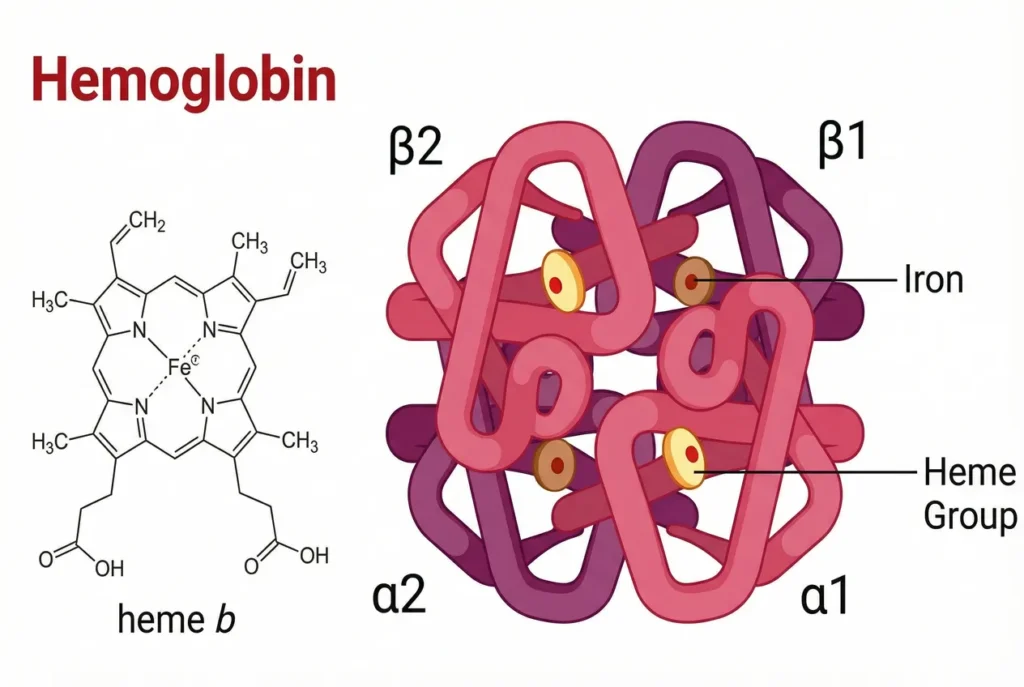
Heme:
The iron-containing heme belongs to the class of chemicals known as protoporphyrin, which is made up of four pyrrole rings that are joined to form the porphyrin ring by a methene (=CH) bridge. The porphyrin ring is connected to four methyl, two vinyl, and two propionate side chain groups. There are fifteen possible configurations for these substitutes. However, protoporphyrin IX is the sole isomer of this compound that exhibits biological activity.
The iron atom has six coordination bonds with the four nitrogen atoms of the protoporphyrin ring, which allow the iron (Fe2+) to be present at the center of the protoporphyrin molecule. The porphyrin ring system’s iron and nitrogen atoms are joined by four connections.
The fifth bond, referred to as proximal histidine, is located between the nitrogen atoms of the histidine trace of the globin polypeptide chain.
The sixth link is created with oxygen. The H-bond between oxygen and the distal histidine, another histidine side chain residue of the globin chain, stabilizes the oxygenated form of hemoglobin.
Globin:
Four polypeptide chains with two distinct fundamental structures (monomeric units) make up globin.
Two alpha (α) chains, each with 141 amino acid residues, and four polypeptide chains, and the types of hemoglobin are two delta (δ), two gamma (γ), or two beta (β). Each of the chains has 146 residues of amino acids.
Hemoglobin Function:
Hemoglobin function in the human body is the following:
(1) Transport of oxygen from lungs to tissues:
Oxyhemoglobin is created when oxygen binds to the heme’s ferrous (Fe²⁺) atom. Oxyhemoglobin has a vivid red color. Hemoglobin becomes fully saturated (loaded) with oxygen in the lungs, where there is a high concentration of O2 (and therefore high pO2). Conversely, oxyhemoglobin releases its O2 for cellular respiration at the tissue level, where the O2 content is low (and thus low pO2). This is frequently accomplished via attaching oxygen to myoglobin, which acts as the tissue’s direct source and storage of oxygen.
A physical process known as oxygenation takes place when oxygen attaches to hemoglobin, producing oxyhemoglobin. In this combination, the iron stays in its ferrous state. Oxyhemoglobin is an unstable substance, and the combination is reversible; that is, it unites with hemoglobin when more oxygen is available, and hemoglobin can easily release oxygen when needed.
(2) Transport of H+ and CO2 from tissues to the kidney and lungs:
Carboxyhemoglobin is created when carbon dioxide is bonded to the N-terminal end of each hemoglobin polypeptide chain. Carboxyhemoglobin is brilliant cherry red in hue. In aerobic metabolism, one CO2 molecule is released for each O2 molecule that is absorbed. The movement of CO2 from the tissues to the lungs is actively facilitated by hemoglobin. About 15% of the CO2 in blood forms a direct bond with hemoglobin. Bicarbonate (HCO3-) is used to transfer the remaining tissue CO2. As seen below, uncharged α-amino acids of hemoglobin are joined with carbon dioxide molecules to generate carboxyhemoglobin.
Hb – NH2 + CO2 = Hb – NH – COO + H+
While deoxyhemoglobin can bind 0.40 moles of CO2/mole heme, oxyhemoglobin can only bind 0.15 moles. The T (taut) type of hemoglobin structure is stabilized by CO2 binding, which lowers the affinity of O2 for Hb. Additionally, hemoglobin facilitates the movement of CO2 as bicarbonate. The erythrocytes’ carbonic anhydrase enzyme catalyzes the creation of carbonic acid (H2CO3) when CO2 enters the blood from tissues. When carbonicacid dissociates, bicarbonate (HCO3-) and a proton (H+) are produced. The proton instantly binds with the carbonic acid. Four oxygen molecules are thought to be released into the tissues for every two protons that are linked to hemoglobin. Protons are created in the lungs when O2 is bound to Hb.
Carbonic acid is created when protons and bicarbonate interact. Carbonic anhydrase reacts with the latter to release CO2, which is then exhaled.
(3) It is involved in acid-base balance because it serves as an intracellular buffer.
The T and R forms of hemoglobins:
The Tense (T) form:
Van der Waals forces, hydrogen bonds, and salt bonds hold the four subunits of hemoglobin together in the deoxyhemoglobin, also known as the tense (T), taut, or stressed form.
The Relaxed (R) form:
- The relaxed (R) form of hemoglobin is the name given to oxyhemoglobin.
- An O2 molecule that is first bound by the α-chain, whose heme pockets are easier to reach than those of the β-chains. Valine residue blocks the β-chains’ heme pockets.
- All four subunits’ salt bonds break when oxygen binds, resulting in the production of protons.
- The secondary, tertiary, and quaternary structures of hemoglobin are altered by these modifications, which also cause the heme pockets of the remaining subunits to expand and make it easier for O2 to attach to them.
- Hence, the deoxyhemoglobin’s stressed, tense, or taut structure converts into the oxyhemoglobin’s relaxed R form.
Types of Normal Hemoglobins:
There are several different types of normal human hemoglobin, and each one has four subunits made up of possibly five different but related polypeptide chains. The majority of normal human hemoglobin is composed of two alpha and two beta chains.
Hb-A1:
Two alpha and two beta chains make up normal adult hemoglobin, often known as Hb-A. In a healthy adult, this form of hemoglobin makes up around 98% of the total.
Hb-A2
It is known as a2d2 and is made up of two alpha and two delta chains. Typically, it accounts for 2.5% of the entire amount.
Hb-A3:
Additionally, there is an electrokinetically rapid fraction that makes up 3–10% of the total.
This is marked as A3, and it looks to be a modified type of Hb-A, mostly present in aged red blood cells.
Hb-F:
The primary hemoglobin present in a pregnancy and a newborn is HbF. It is made up of two alpha and two gamma chains. Following birth, beta chains take the place of the gamma subunits, resulting in 2b2 (HbA1).
Hb-M:
The alterations in the histidine residue of either- or -chains that bind to the iron in the heme molecule are the cause of these.
• Tyrosine replaces histidine in HbM, and iron is stabilized in the ferric (Fe3+) form rather than the ferrous (Fe2+) form, which prevents oxygen binding and causes cyanosis.
• The afflicted chains are in the methemoglobin form, as indicated by the letter “M” in HbM.
Glycosylated Hemoglobin:
Glycosylated hemoglobins (HbA1C) is a byproduct of the spontaneous reaction between adult hemoglobin (HbA1) and glucose.
- When blood glucose enters the erythrocytes, its aldehyde group reacts nonenzymatically with the valine, the N-terminal residue of β chains.• Typically, blood’s HbA1C concentration is extremely low, making up only around 5% of total hemoglobin.
• The production of HbA1C is correlated with blood glucose levels.
• Patients with diabetes mellitus, a condition in which blood sugar levels are elevated, have higher levels of HbA1C.
• Since red blood cells only last for 120 days, measuring HbA1C gives valuable information for managing diabetes mellitus. It shows how well blood glucose levels have been controlled during the preceding two to three months.
Types of abnormal hemoglobin:
Because hemoglobin’s ε, ε, ã, and ⃤ chains are genetically assembled from amino acids, mutations in the genes encoding these chains can disrupt hemoglobin’s biological function and development. We refer to this type of hemoglobin as aberrant hemoglobin. Hemoglobinopathy is the term for a disorder in which a mutation in hemoglobin alters biological functioning. Below is a discussion of some of the aberrant hemoglobin types.
Thalassemia:
Thalassemia is a collection of genetically transmitted hemoglobin-related disorders caused by reduced or absent α or β globin chain production. It is an autosomal recessive genetic condition. The other globin chains are produced in relative excess while the production of one globin chain is decreased. The cell may experience the precipitation of these globin chains, leading to hemolysis and hypochromic anemia.
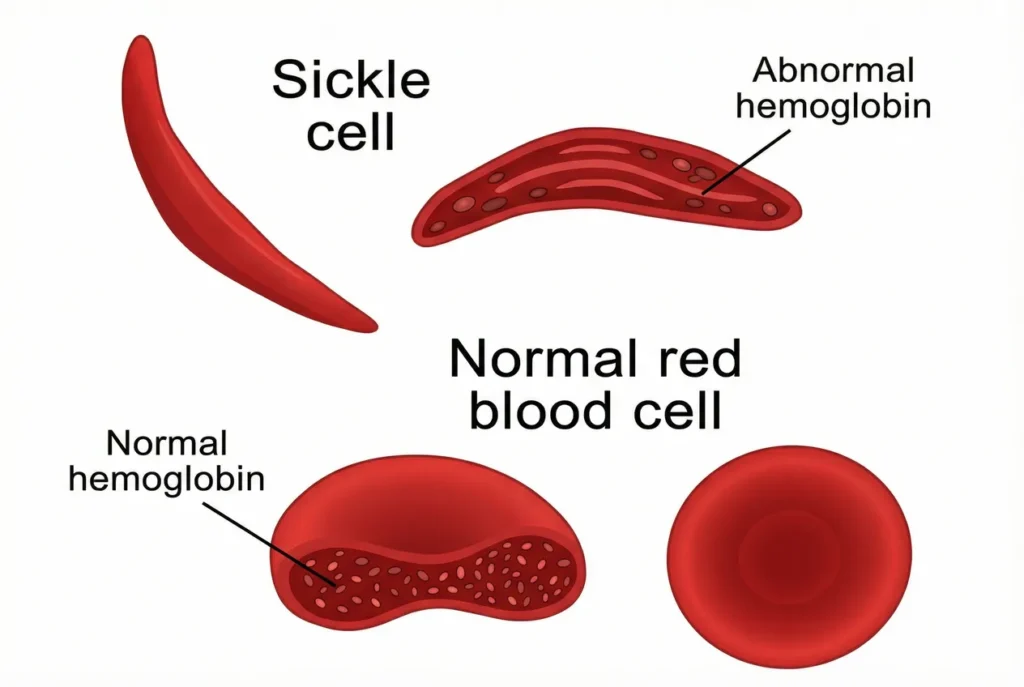
Sickle Hemoglobins[Hbs]:
- Sickle cell anemia is a hereditary condition caused by sickle hemoglobin (HbS), an aberrant form of hemoglobin.
- A mutation in the β-globin gene, which codes for the ε-globin chain, causes the production of HbS. The amino acid composition of the HbS mutant α-globin chain is changed.
- A mutation in the -globin chain results in the substitution of a valine residue for the glutamic acid residue that was previously present in the sixth position of the -chain of HbA.
- Due to a mutation in the θ-globin chain, a valine residue takes the place of the glutamic acid residue that was previously present in the sixth position of the ε-chain of HbA.
- When nonpolar (hydrophobic) valine replaces polar (hydrophilic) glutamic acid, a hydrophobic contact point known as a “sticky patch” is created on
the outermost portion of the -globin chain. This mutation is significant. - The red blood cells, which resemble a sickle blade, are deformed due to the insoluble fibers of deoxygenated hemoglobin S. Thus, the illness is known as sickle cell disease.
- Due to their misplacement of water, sensitivity, and significantly shorter lifespan (17 days versus 120 days) than normal cells, sickled red blood cells cause lysis of the red blood cells and causes sickle cell anemia, also known as hemolytic anemia.
- The most severe reaction is that the lengthy, atypically shaped red cell blocks tiny blood capillaries in several organs. This excerpt from
the oxygen supply and results in anoxia, or oxygen scarcity, which hurts and ultimately kills the cells.