Base catalyzed rearrangement in which α-halo ketone or cyclopropane having at least one α-H reacts with base give carboxylic acid or their derivatives.
In favorskii rearrangement, the base decides our product e.g. if alkoxide is used as a base then an ester will be formed.
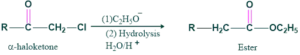
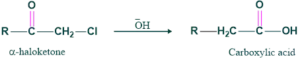
If hydroxide is used as a base then carboxylic acid will be formed.
If amine is used as a base then amides will be formed.
Favorskii Rearrangement Reaction:
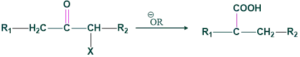
Mechanism of Favorskii Rearrangement:
The mechanism of favorskii rearrangement involves 4 step
(1) Formation of carbanion
(2) Formation of cyclopropane(intermediate is highly unstable)
(3) Attack of nucleophile
(4) Protonation
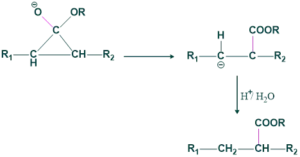
Step(01) Formation of Carbanion:
In this step, base attack on α-H and take this H to form a carbanion.

Step(02) Formation of cyclopropane( Intermediate):
In this step, the attack of intramolecular nucleophiles on the carbon-bearing halogen atom forms a volatile symmetrical cyclopropane ring.

Step(03)Attack of nucleophile:
In this step, an attack of nucleophiles on the carbonyl carbon takes place which opens the ring, so some type of product is obtained because ketone is symmetrical.

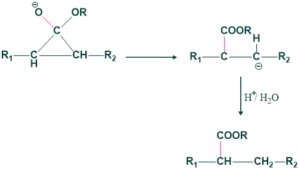
Step(04)Protonation:
Here possibilities take place
(a) Bond breaking from the left side.
(b) Bond breaking from right side.
Cyclic Ketone:
Favorskii rearrangement involves ring contraction;
Reaction:
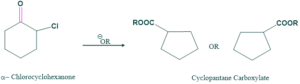
Mechanism:
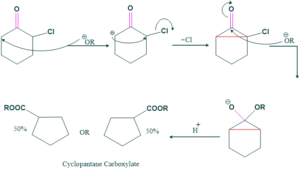
Application of Favorskii Rearrangement:
- Preparation of carboxylic acid
- Preparation of unsaturated compound from di-halo ketone
Preparation of carboxylic acid:
If hydroxide is used as a base then carboxylic acid is formed as follows;
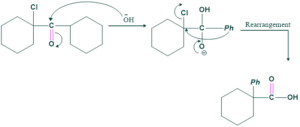
In this compound, no α-H is present. So we do not from enol, we directly attack OH, and shifting takes place and gives our final product.
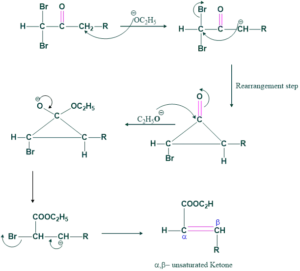
Preparation of unsaturated compound from di-halo ketone:
Dihaloketone may be vicinal or geminal
(a) using a vicinal
Reaction:

Mechanism:
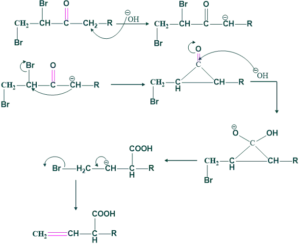
(b)Using a geminal:
Reaction:
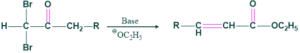
Mechanism: