What is conjugation in chemistry?
- The term “conjugated” was coined in 1899 by the German chemist Johannes Thiele.
- The phenomenon of overlap of p-orbitals with delocalized electrons in a molecule is called conjugation.
- It generally decreases the overall energy of the molecule and increases the stability of the molecule.
- It is the overlap of p-orbitals across a σ bond (sigma bond) .
- This phenomenon occurs between two p orbitals.
- It occurs in compounds having alternating single and double bonds.
- It results in a delocalized pi electron cloud.
Examples of conjugation:
Four examples of conjugation are given below:
1. Conjugation with empty p-orbital of carbocation:
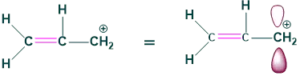
2.Conjugation with lone pair on nitrogen:
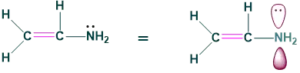
3.Conjugation with another pi bond:

4.Conjugation with a radical:

Recommended video: