What is cholesterol?
- Cholesterol is the sterol of the higher animals, which occur as free or as fatty esters in all animal cells especially in brain and spinal cord
- It was first isolated from human gall stones. Gall stones contains almost entirely of cholesterol.
- It is a white crystalline solid which is optically active . It shows levo rotation.
- It’s melting point is 149°.
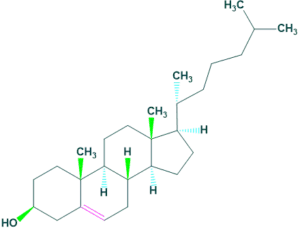
Structure of cholesterol:
 Sources of cholesterol:
Sources of cholesterol:
The main sources of cholesterol are the fish -liver oils, and the brain and spinal cord of cattle.
- Lanoline is the fat from wool that is a mixture of cholesteryl palmitate, stearate and oleate.
Identification tests:
Cholesterol and many other sterols gives many color reactions which are given below:
The Salkowski reaction:
When concentrated sulphuric acid is added to a solution of cholesterol in chloroform, a red color is produced in the chloroform layer.
The Liebermann-Burchard reaction:
A greenish color is developed when a solution of cholesterol in chloroform is treated with concentrated sulphuric acid and acetic anhydride.
- When an ethanolic solution of cholesterol is treated with an ethanolic solution of digitonin then white precipitates of cholesterol digitonin is formed. This is a molecular complex containing one molecule of cholesterol and one of digitonin. The components may be recovered by dissolving the complex in pyridine ( which brings about complete dissociation ) and then adding ether ( the cholesterol remains in solution and the digitonin is precipitated). The formation of digitonin is used for the estimation of cholesterol.
Structure elucidation of cholesterol:
1.Molecular Formula:
The molecular formula of cholesterol is C27H46O.
2. Double bond equivalent:
It’s double bond equivalent is 5.
3. Identification of nature of oxygen:
- When cholesterol is treated with 2,4-dinitrophenyl hydrazine , no product is formed. It indicates that carbonyl is absent so oxygen nature is not carboxylic.
![]()
- When cholesterol is treated with acetic anhydride in the presence of pyridine ,product is formed which indicates that oxygen nature is alcoholic.
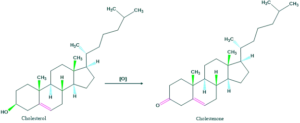
- When cholesterol is oxidized then keto product is formed which indicates that hydroxyl nature is secondary.
4. Unsaturation:
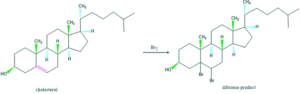
- It was treated with bromine which gave dibromo adduct. It confirms the presence of one double bond.
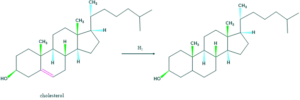
- The presence of one double bond in cholesterol is confirmed by hydrogenation reaction. One mole of hydrogen occurs which indicates the presence of one double bond.
- Therefore, 1 D.B.E = 1 double bond and 4 D.B.E = 4 Rings
5. Ring Size determination:
The size of the ring is determined by following the Blank’s rule.
Blanks rule:
- It states that if a compound is dicarboxylic acid and they are separated by 1,2,3 carbon atoms, then that compound on heating with acetic anhydride will a cyclic anhydride.
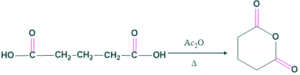
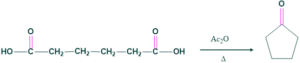
- If two carboxylic groups are separated by more than three carbon atoms that compound on heating with acetic anhydride will give a cyclic ketone with the loss of one carbon atom..
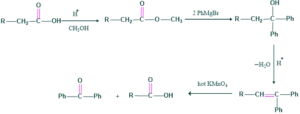
Barbier weiland degradation:
The Barbier-Wieland degradation is a process that reduces the carbon chain of a carboxylic acid by one carbon. It only works when the carbon next to the carboxyl is a simple methylene bridge (an aliphatic carbon without any substituents). The carboxyl and alpha carbon are converted into an alkene, which is subsequently oxidized to turn the former alpha position back into a carboxyl.
By using Blanks rule ,size of rings present in cholesterol are determined.
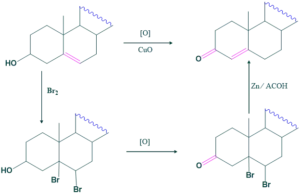
Ring A:
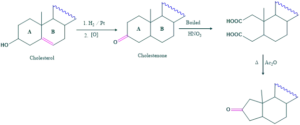
Cholesterol on reduction form cholestanol which on oxidation form cholestanone. When cholestanone is further oxidized it forms dicarboxylic acid which on acetylation form cyclopentanone derivative which indicates that it is 1,6-dicarboxylic acid. So cholesterol must have a cyclohexane ring called as ring A.
Ring B:
When 5α-cholestro-6-one is oxidized it forms dicarboxylic acid which on acetylation form cyclopentanone derivative which indicates that it is 1,6-dicarboxylic acid. So, cholesterol must have a cyclohexane ring called as ring B.
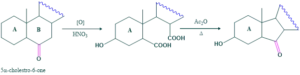
Ring C:
When 12-oxodeoxycholic acid is oxidized it forms dicarboxylic acid which on acetylation form seven membered anhydride instead of formation of cyclic ketone which exhibits failure of Blanks rule.
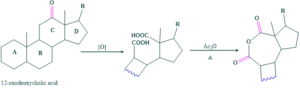
Ring D:
5β-cholonic acid on Barbier-wieland degradation form cyclopentanone derivative which on oxidation form dicarboxylic acid . When this dicarboxylic acid is heated with acetic anhydride , a seven membered cyclic anhydride is formed.
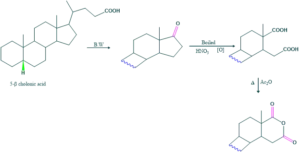
Determination of position of hydroxyl group:
When cholesterol on reduction form cholestanol but on further oxidation it form cholestanone. On oxidation of cholestanone ,two compounds of dicarboxylic acids are formed.
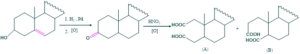
The formation of (A) dicarboxylic acid compounds exhibit that CO is flanked by the two methylene groups as
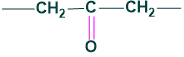
So, hydroxyl group is present only in (A) ring.
Methyl group confirm the position of hydroxyl group because methyl group added at that carbon which contain OH group and keto group is obtained by the oxidation of hydroxyl group.
Determination of position of double bond:
Cholesterol on its oxidation in the presence of hydrogen peroxide converted into triol. Two hydroxyl groups are converted into keto group by oxidation but one is resist to oxidation which shows that OH group is tertiary and double bond may be at junction.
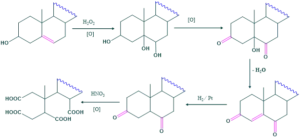
This tetracarboxylic acids product shows that two keto groups are in two different rings.
λmax of α,β-conjugated cyclohexanone derivative is 240 nm. This is detected by UV spectroscopy. This shows that double bond is at 5 and 6 position but by reaction it is at 4 and 5 position.
Determination of position of side chain:
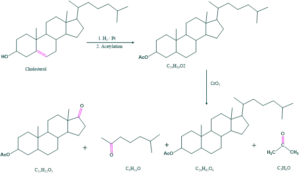
Formation of acetone indicates that isopropyl is terminated part of side chain.
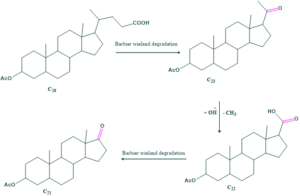
The barbier-weiland degradation shows that the side chain contains eight number of carbon atoms.
Determination of position of angular methyl group:
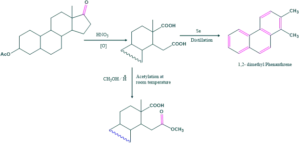
Only one group is esterified which was primary . While the other -COOH group found to be tertiary which resist to esterification. This indicates methyl group is present here.
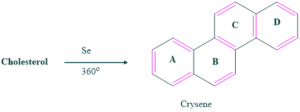
The structure of crysene is determined by X-ray crystallography. Ring D is six membered, formation of six membered ring indicates that one methyl group is present which has become part of 5 membered ring.
![]()
This -COOH group is not esterified and not subjected to Barbier Weiland degradation indicate that one methyl group is present here.
Stereochemistry of Hydroxyl groups:
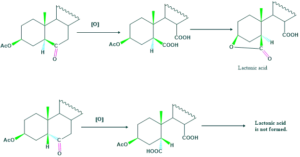
In case when hydroxyl group is on β position, lactonic acid is formed. While in case when hydroxyl group is on α position, no formation of lactonic acid.
This discussion shows that configuration of Hydroxyl group is “β”.
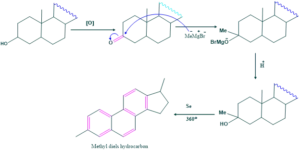
Synthesis of cholesterol:
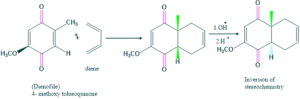
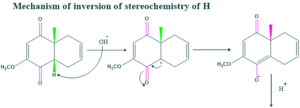
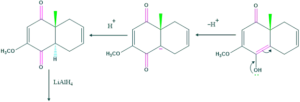
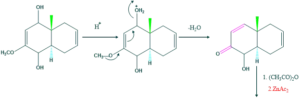
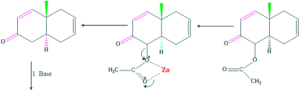
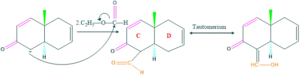
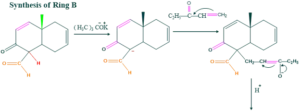
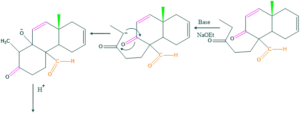
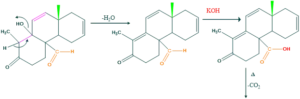
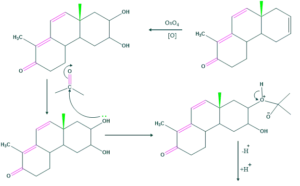
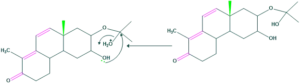
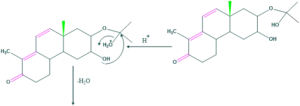
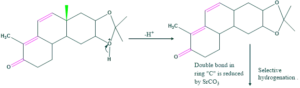
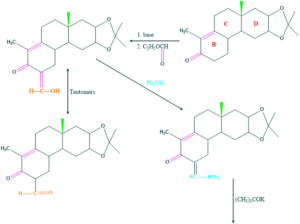
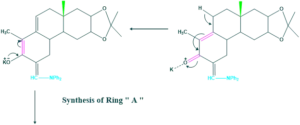
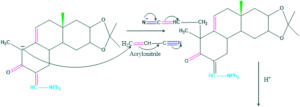
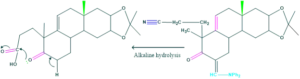
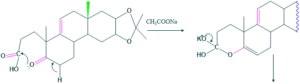
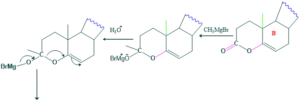
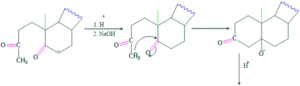
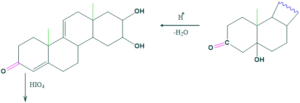
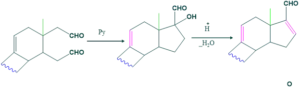
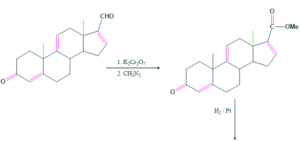
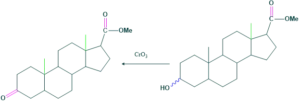
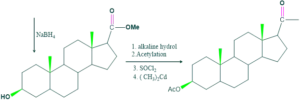
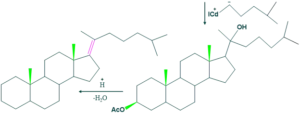
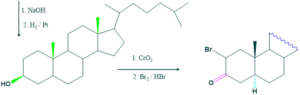
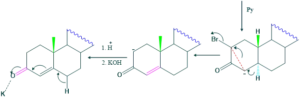
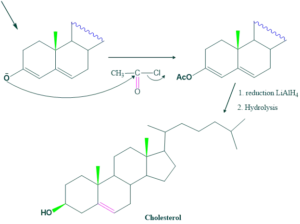
FAQ
What is biggest factor in cholesterol?
An unhealthy lifestyle is the leading cause of high “bad” LDL cholesterol and low “good” HDL cholesterol. However, genes inherited from your parents, various medical problems, and some medications may elevate LDL cholesterol levels or lower “good” HDL cholesterol levels.
Which organ is the biggest producer of cholesterol?
Your liver produces all the cholesterol that is needed by your body. Lipoproteins, which are spherical particles, carry cholesterol and other lipids through the bloodstream. The two most well-known lipoproteins are low-density lipoproteins (LDL) and high-density lipoproteins (HDL).
Which hormone is formed from cholesterol?
All steroid hormones, including glucocorticoids, mineralocorticoids, and sex hormones, are derived from cholesterol. The placenta and ovaries produce estrogens and progestins, whereas the testes produce testosterone and the adrenal cortex produces cortisol, aldosterone, and androgens.
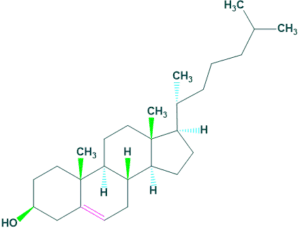 Sources of cholesterol:
Sources of cholesterol: