Cholesterol
What is cholesterol? Structure of cholesterol: Sources of cholesterol: The main sources of cholesterol are the fish -liver oils, and…
The branch of science which deals with the composition ,structure, properties and reactions of matter. There are different branches of chemistry like organic chemistry, inorganic chemistry, analytical chemistry biochemistry, physical chemistry, industrial chemistry, nuclear chemistry
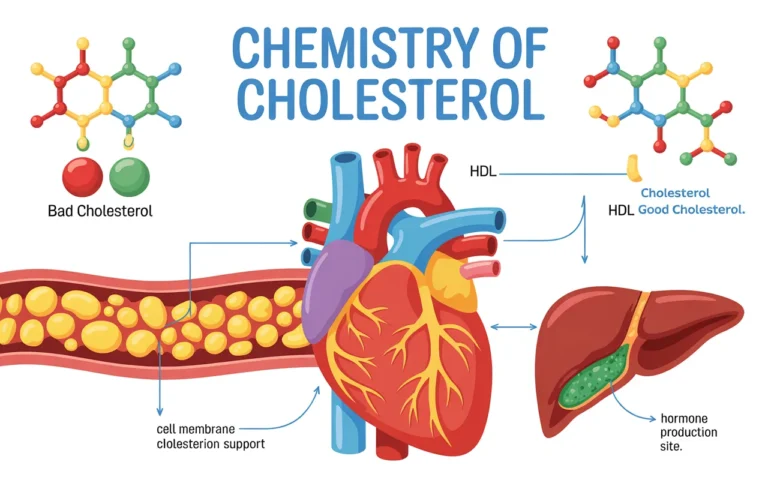
What is cholesterol? Structure of cholesterol: Sources of cholesterol: The main sources of cholesterol are the fish -liver oils, and…
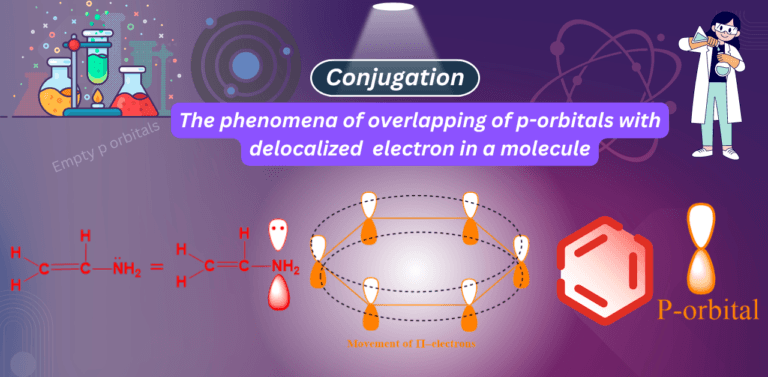
What is conjugation in chemistry? Examples of conjugation: Four examples of conjugation are given below: 1. Conjugation with empty p-orbital…
Benzil-Benzilic Acid Rearrangement Base-catalyzed reaction in which 1,2-diketone reacts with a hydroxyl ion to give a hydroxyl carboxylate ion, which…
What are steroids? Classification of steroids: 1. Sterol: These core structure of steroid ( C-17) with the aliphatic side chain…
What is citral? Chemical formula: the chemical formula of citral is C10H16O Boiling point : Its boiling point is 77…
What are steroid hormones? A steroid hormone is a steroid that functions as a hormone. Steroid hormones are a class…
What are terpenoids? What is the difference between terpenes and terpenoids? What is isoprene? According to the isoprene rule proposed…

Sugar Industry The sugar industry produces one to two products and a number of byproducts, as its main function is…
ABO Blood group ABO blood group system is primarily a blood type classification system. ABO blood group test is a…
Carbocations Carbocations are the most important reactive intermediate in many kinds of reactions, and nucleophilic substitution is one of them….