[ez-toc]
Carbocations are the most important reactive intermediate in many kinds of reactions, and nucleophilic substitution is one of them. These carbocations have a special place in organic chemistry because of many factors, like their rearrangements to gain stability, etc.
Structure and hybridization of carbocation:
The central carbon of carbocation is sp2 hybridized because one s and two p orbitals are involved in hybridization, and the empty p orbital remains perpendicular to the rest of the molecule. Carbocations are planar in structure with a 120º angle between atoms.
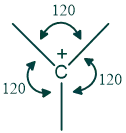
What are the rules for the stability of carbocation?
- If there is an electron-donating group present along with carbocation, then it will make carbocation stable by compensating its electrons.
- If an electron-withdrawing group is present, it will reduce the stability of carbocation by further reducing its electron density.
- Hyperconjugation plays an important role in this regard because alpha hydrogen can overlap its electrons with carbocation, and then the deficiency of carbocation will be compensated, and it will stabilize.
- The type of solvent plays an important role in this regard because polar protic solvents have dipole-dipole interactions, which will have a delta (-ve) charge. This negative charge will give electrons to empty the p-orbital of carbocation and stabilize it.
- The most important factor in the stability of carbocation is resonance; this effect has the highest weightage and has been given the highest priority factor in stabilizing carbocations. Delocalization due to the resonance effect can increase the stability of carbocation.
Why are tertiary carbocations more stable than secondary or primary carbocations?
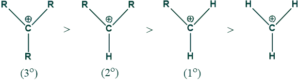
Reason:
As methyl groups have a +I effect and show a hyperconjugation phenomenon, so more methyl groups, the more stable the carbocation will be. That’s why tertiary carbocation is more stable than secondary carbocation, which is more stable than primary carbocation.
Why tertiary phenyl carbocations are less stable species?
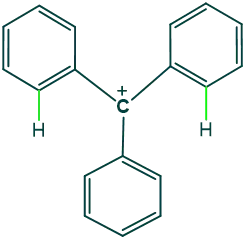
Reason:
Tertiary phenyl carbocation has 3 phenyl groups that are closer to each other, due to which they exert van der Waal’s repulsion on each other; because of this phenomenon, rings start tilting concerning each other, and they do not remain in the same plane. This is the reason that they do not show such resonance as seen in other highly conjugated systems. So, they cannot provide enough electron density to carbocation, making it less stable.
Aromaticity of carbocations:
Aromatic carbocations are more stable than non-aromatic and anti-aromatic carbocations because the aromatic system provides stability by providing electrons to carbocations.
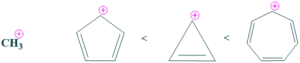
- Cycloheptatrienylium ion is the most stable because it is aromatic and also has a resonance-stabilized system.
- Cyclopropenylium ion is on the second number because it is aromatic.
- Cycloheptadienylium ion is on the third number because it is anti-aromatic.
Why are tertiary cyclopropyl carbocations more stable species?
Tertiary carbocations have three cyclopropyl rings that are stable because of a special type of bond between them called a banana bond. Cyclopropyl ring has a hydrogen atom that is tilted towards the carbocation which provides more electron density than any other system which makes carbocation more stable.

The greater the number of cyclopropyl rings, more will be the stability of carbocations due to tilted hydrogen bonds; that’s why tricyclopropyl carbocation is more stable than dicyclopropyl carbocation, which is more stable than monocyclopropyl carbocations.