Reactive intermediates
Intermediates are the type of species that formed during a chemical reaction. They are short-lived species with low stability, but their stability may increase by further rearrangements. In any chemical reaction, almost two types of intermediates are formed:
- Synthetic intermediates
- Reactive intermediates
What are synthetic intermediates?
Synthetic intermediates are our main products, they are intentionally prepared and purified. These synthetic intermediates may work as starting material in the formation of another product.
What are reactive intermediates?
are less stable than synthetic intermediates, they have a very short period during which they convert into another type of molecule or may transform another molecule into an unstable species by stabilizing itself (by gaining or losing electrons).
There are many types of reactive intermediates:
- Carbocation
- Carbanion
- Free radicals
- Carbenes
- Nitrenes
- Arenium ions
- Benzynes
What are Carbocations?
- Carbocations are sp2 hybridized.
- They have a planar structure.
- Bond angle of about 120 degrees
- Vacant p orbital is perpendicular to the other planar bonds of sp2 carbon.
- Carbocations are electron-deficient species because they have a deficiency of 2 electrons in their outermost shell.
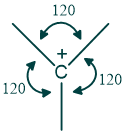
⇒ Carbocations are present in different forms they may be
- Bridged non-classical
- Bridgehead carbonium ions
Stability of carbocation:
- The greater the inductive effect, the greater will be the stability of carbocation.
- If there is an -I effect, then carbocation will be destabilized.
- If there is a +I effect, then carbocation will be stabilized.
- Hyperconjugation plays an important role in the stability of carbocation.
- The greater will be the alpha hydrogens, the greater will be the stability because hyperconjugation will stabilize the carbocation by overlapping electrons.
- Resonance also plays an important role in stabilizing the carbocation by donating electrons through pi bonds by delocalization.
What is carbanion?
- Carbanion are sp3 hybridized
- They are pyramidal in structure
- They have a bond angle of about 109 degrees
- Carbanions are electron-rich species
- They may act as nucleophiles or base
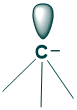
Carbanions are in equilibrium in two umbrella forms. when a leaving group leaves the molecule then the attacking electrophile should attack from the same side, but the coming electrophile is seem attacking from both sides. This is because of the equilibrium that is created between two umbrella forms of nucleophiles.

What are free radicals?
The central carbon of free radicals may be sp2 or sp3 hybridized. They have one unpaired electron in their outermost shell, due to this reason they are paramagnetic. Electron spin resonance can easily detect free radicals. they are an electron-deficient species because they possess seven electrons in their outer shell.
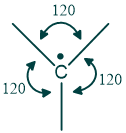
In sp2 type structure bonds are 120 degrees apart from each other.
What are carbenes?
Carbenes are bivalent species that are attached to two groups and contain one lone pair of electrons. They do not carry any positive or negative charge as they are neutral species. Carbenes may be of two types:
- Singlet carbenes
- Triplet carbenes
Singlet carbenes have both nonbonding electrons in the same orbital with opposite spins whereas Triplet carbenes have nonbonding electrons in different orbitals with the same or parallel spins.