Disaccharides:
Among the oligosaccharides, disaccharides are the most common carbohydrates. It consists of two monosaccharide units. Two monosaccharides are held together by a glycosidic bond. Examples of disaccharides are maltose, sucrose, and lactose.
These are crystalline, water-soluble, and sweet. There are two types
- Reducing disaccharides
- Non reducing disaccharides
Reducing Disaccharides:
Reducing disaccharides with a free aldehyde or keto group e.g. maltose, lactose.
Maltose
Maltose is composed of two α-D-glucose units joined by glycosidic linkage between C-1 (anomeric carbon) of one glucose unit and c-4 of the other glucose unit.
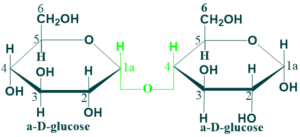
Maltose is a reducing disaccharide because free anomeric carbon of the second glucose unit which
can act as a reducing agent. Maltose is produced as an intermediate product in the digestion of starch and glycogen by the action of the enzyme α-amylase.
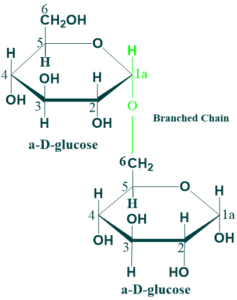
Isomaltose:
In isomaltose, the two glucose units are held together by α (1→4) glycosidic linkage. Isomaltose is a disaccharide derived from the digestion of starch or glycogen. It is hydrolyzed to glucose in the intestinal tract by the enzyme isomaltase.
Cellobiose:
Cellobiose is another disaccharide, identical in structure to maltose, except that the former has β (1→4) glycosidic linkage. Cellobiose is formed during the hydrolysis of cellulose.
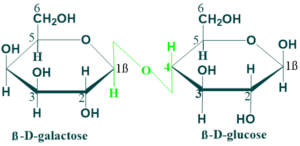
Lactose:
Lactose is present in milk. Lactose contains one unit of β–galactose and one unit of β-glucose that are linked by a
β(1→4) glycosidic linkage. The anomeric carbon of glucose unit is available for oxidation and thus lactose is a reducing disaccharide. Lactose is hydrolyzed to glucose and galactose by lactase enzyme in human beings. Lactose milk is the most important carbohydrate in the nutrition of young mammals.
Non Reducing Disaccharides:
Non-reducing disaccharides with no free aldehyde or keto group e.g. sucrose
Sucrose:
Sucrose is a disaccharide of glucose and fructose. It is formed by plants but not human beings. Sucrose is an intermediate product of photosynthesis. sucrose is also known as table sugar. The structure of sucrose consists of α-D-glucose and β-D-glucose. The two monosaccharides are held together by a glycosidic bond (α→β) between the C1 of the α glucose and C2 of β fructose.
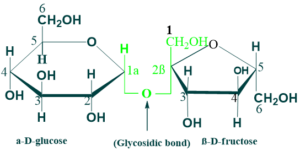
The reducing groups glucose and fructose are involved in glycosidic bonds, hence sucrose is a non-reducing sugar and it can not form osazones. The intestinal enzyme sucrase hydrolyses sucrose to glucose and fructose which are absorbed.
Biomedical importance:
A variety of food preparations, including newborn and invalid foods, are made by hydrolysis of grains that contain high maltose content. From a dietary perspective. Consequently, they are simple to digest.
The lactose is synthesized in the lactating mammary gland for the baby, lactose from breast milk and glucose produced by the duct epithelium provide a good source of energy for newborns.
Typhoid bacillus, which is pathogenic (lactose nonfermenter), does not ferment lactose; instead, “Coliform” bacilli (E. coli), which are often non-pathogenic (lactose fermenter). The purpose of this test is to differentiate between these two microbes.
“Souring” of milk: Several microorganisms, including Str. lactis, A. aerogenes, and E. coli, convert the lactose in milk to lactic acid (LA), which is what causes the souring of milk.
Pingback: Define carbohydrates, Function and Classification - Chemistwizards.com
Great insights! I found your take on sustainable living incredibly practical. Looking forward to implementing some of these tips! Check out [Get Info](https://getinfo.ink/?utm_source=google&utm_medium=search&utm_campaign=promotion) for more inspiring content.