What is lipids?
Lipids are organic heterogeneous compounds found in living organisms and are long chains of triglycerides of fatty acids. These qualitative tests for lipids are used to analyze the properties of fats and oils. Here’s what they’re used for:
 Qualitative tests for lipids[/caption]
Qualitative tests for lipids[/caption]
Acid Value
The test in which the number of mg of alkalis or NaOH\KOH is used to neutralize 01 gram of fats and oils is called the acid number and by dividing it the gain value is called the acid value
Significance:
- Measure the amount of free fatty acids present in fats or oils
- Indicates the degree of rancidity (oxidation of unsaturated fatty acids leading to off flavor and odor) or hydrolysis ( breakdown of triglycerides into free fatty acids and glycerol)
- Used in quality control, processing, and storage of fats and oils
- used to identify the higher AV that indicates more free fatty acids present in a product that can affect the flavor, texture, and shelf life of the product
- 03 moles of alkalis are required to neutralize one mole of oil and after neutralization color will be changed
EXPERIMENTAL ANALYSIS
The processes that we used in the laboratory mostly are given below
Principle
Used to determine acid value by titrating the fat or oil against KOH or NaOH solution using phenolphthalein indicator in which alkalis react with free fatty acid present instead of breaking the ester bond.
Requirements
- conical flask
- burret
- measuring flask
- stirrer
- thermometer
- iron stand
- tripod stand
- beakers
- pipette
- sample solution (95% ethanol and etherin equal amount)
- phenolphthalein indicator ( dissolve 1g of phenolphthalein in 100ml of ethyl alcohol)
Procedure
- Add 10g of analyte or sample to a conical flask.
- Add 50ml of eq solution of ethanol and ether then add 1-2 drops of phenolphthalein indicator
- now titrate the solution with one molar KOH/NaOH Solution with the help of burret
- Add alkali until neutralization (color change) occurs
- take a reading from burret
Formula
Acid value = (volume of alkali used). (molarity of alkali) .(molar mass of alkali)/weight of the sample
Result
The color will be changed from transparent or white to pink in this qualitative test for lipids.
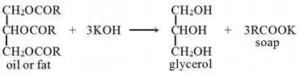
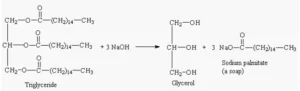
Saponification value
The number of mg of NaOH required to saponify one gram of fats/oils is called the saponification number and after dividing it, the value is called the saponification value.
Significance:
- Measure the amount of fatty acids present in fats or oils including both free and bounded
- indicates the average molecular weight of fatty acids
- Higher SV indicates more fatty acids which can affect soap-making, lubrication, and other industrial applications
- used in the production of soap, detergents, cosmetics, and lubricants.
Types of Titration
There are two types of titration
- Sample titration
- Blank titration
Sample Titration
The type of titration in qualitative tests for lipids in which we use samples is called sample titration.
In this process firstly we add an excess amount of KOH to a known quantity of sample for soap formation in a conical flask then add an indicator. After saponification, the remaining KOH is determined by titrating it against a standard acid HCl. Note the reading on burret.
Blank Titration
In this titration, no sample is used, water is added to replace the sample therefore called blank titration.
Another process is the same as sample titration, add excess KOH in a conical flask, add a few drops of phenolphthalein indicator, and then add HCl for titration.
EXPERIMENTAL ANALYSIS
Principle
This titration is used for the determination of saponification value and fatty acids.
- It indicates the length of the carbon chain of fatty acids, higher the saponification value, the shorter the acid chain present in fat oil
- Also gives an idea about average molecular weight, higher S.V than lower weight
S.V= 1/weight
Requirements
Similar to the apparatus used for the determination of acid value, we can use it in identifying the saponification value
Procedure
- Add the sample to a conical flask
- add an excess amount of KOH
- Add a few drops of indicator
- Add HCl to titrate the remaining KOH
- the last step in sample titration is to read the readings
- Prepare blank solution take water in place of sample in conical flask add excess KOH, then a few drops of indicator then HCl and calculate the readings
- Readings on blank solution will be high so readings of sample titration will be minus
Formula
S.V = ( molar mass of KOH).( molarity of HCl).(BHCl -SHCl) / weight of the sample
Result
The solution will be colorless
Ester value
The determination of the ester bond between fatty acids is called the ester value. It identifies the difference between saponification and value.
Formula
E.V = S.V – A.V
Significance
- In the analysis of fat or oil, ester value is significant, particularly in industries like food and cosmetics where sensory characteristics of the product are crucial
- A higher value may indicate a higher concentration of flavor and fragrance
free fatty acids = 1 / fragrance
- used to determine all fatty acids (free +fatty acid in bound form)
Iodine value
The number of mg of iodine absorbed by 100g of fat/oil is called the iodine value.
It measures the degree of unsaturation in lipids. Each fat/oil has its specific iodine number.
Equation
![]()
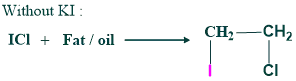
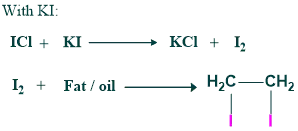
Significance
- iodine value measures the degree of unsaturation of oil/fat
- The greater the value of iodine more unsaturation and higher susceptibility of oxidative rancidity
- Lower iodine number, oil will be more desirable
EXPERIMENTAL ANALYSIS
Chemical
- CCl4
- sample solution
- excess amount of iodide monochloride
- glacial acetic acid
- sodium thiosulphate/ potassium iodide
- starch solution
- indicator
Apparatus
- Beakers
- conical flask
- round bottom flask
- thermometer
- stirrer
- iron stand
- tripod stand
- measuring flask
- pipette
Procedure
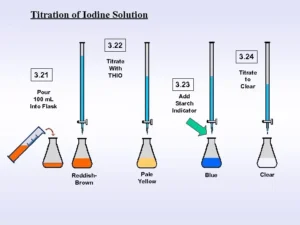
- Iodine value can be determined by the Wijis method
- Make a solution of CCl4 and sample then titrate with an excess amount of iodine monochloride ICl solution into glacial acetic acid
- now remaining iodine monochloride is treated with sodium thiosulphate or potassium iodide
- The centrifugation technique is used to mix well
- starch solution is used as an indicator
Types
There are two types of titration samples and blank. Blank titration is the same as sample titration but without sample and titration process starts. The blank solution has a higher value than the sample solution.
Formula
Iodine value = [(amount of sodium thiosulphate in Blank sol)- ( amount of Na2S2O3 in sample )]. (molarity of Na2S2O3) ( molar mass of Na2S2O3 and potassium iodide ) / weight of the sample
Result
The solution will be converted into colorless or transparent.