What is Michael’s Addition?
Table of Contents
ToggleMichael addition reaction is also called the 1,4 addition reaction or Conjugated addition. When Michael donor ( ketone, aldehyde, cyano group, or other nucleophile ) reacts with Michael acceptor ( α,β- unsaturated carbonyl compound ), it forms a C-C-β carbonyl compound or Michael adduct. α, and β-unsaturated carbonyl compounds usually have electrophilic double bonds. The β carbon is electrophilic because it shares the positively charged carbonyl carbon through resonance.
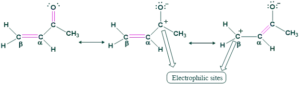 A nucleophile can attack an α,β-unsaturated carbonyl compound either at the carbonyl group or at the β position. When attack occurs at the carbonyl group protonation of oxygen leads to a 1,2-addition product in which nucleophile and proton leads have added to adjacent carbon atoms. When an attack occurs at the β position, the oxygen atom is the fourth atom counting from nucleophile and the addition is 1,4-addition. This reaction was given by Arthur Michael in 1887, of Tufts University and later Harvard University.
A nucleophile can attack an α,β-unsaturated carbonyl compound either at the carbonyl group or at the β position. When attack occurs at the carbonyl group protonation of oxygen leads to a 1,2-addition product in which nucleophile and proton leads have added to adjacent carbon atoms. When an attack occurs at the β position, the oxygen atom is the fourth atom counting from nucleophile and the addition is 1,4-addition. This reaction was given by Arthur Michael in 1887, of Tufts University and later Harvard University.
Michael Addition Reaction:
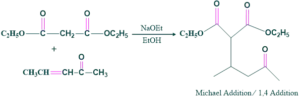
Michael Addition Mechanism:
Step(01) enolate formation:
In this step ethoxide base attacks on the diester to form an enolate ion. It means the ethoxide base deprotonates α-carbon of a carbonyl compound to form a nucleophile or Michael donor which is a strong nucleophile.

![]()
Step(02) nucleophile attack:
In this step a nucleophile ( Michael donor) attacks on Michael acceptor which is 3 penten-2-one and forms an adduct. Enolate ion attacks the β-carbon of the α,β-unsaturated carbonyl compound. The double shifts create a new single bond between nucleophile and β-carbon, while the carbonyl group remains intact.

Step(03) protonation
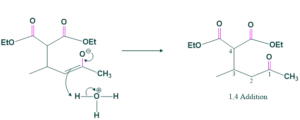
In this step acid attacks on adduct and gives 1,4 additional products or conjugated products. The intermediate formed after the nucleophile attack has a negatively charged oxygen atom ( in the case of an enolate ion) or a negatively charged carbon atom ( in the case of another nucleophile). This intermediate is then protonated either by the solvent or by the conjugate acid of the base used, leading to the final product.
Important Note for Problem Solution:
Claisen condensations usually give 1,3-dicarbonyl products with one saturated carbon between two groups. Conjugate addition commonly gives 1,5-dicarbonyl products with three saturated carbons between two carbonyl groups. When you need a compound with three carbons between two carbonyl groups, consider a conjugate addition.
Example of 1, 4 Addition Reaction:
Dimethyl melonate reacts with cyclohexanone giving the following product.
Attack of Base:

![]()
Nucleophilic Attack:
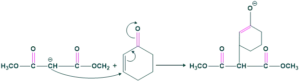
Acid Attack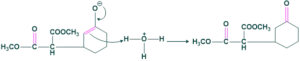
Application of 1,4 Addition Reaction:
Pharmaceuticals
In the synthesis of pharmacological compounds, 1,4 addition reactions are frequently employed when certain functional groups must be added in a controlled way.
Natural Product Synthesis:
The synthesis of natural products like steroids and alkaloids often involves this reaction.
Factor Affecting the Michael Addition:
Selection of nucleophile:
The nucleophile’s reactivity is important. The most frequently used nucleophiles are strong ones, which include enolates. Under suitable conditions, weaker nucleophiles like amines or thiols can still take part in the process.
Versatility:
The Michael addition is not limited to enolates; various nucleophiles like amines, thiols, and stabilized carbanions (like malonates and cyanoacetates) can participate, broadening the scope of the reaction.
Nature of Electrophile:
To increase the electrophilicity and responsiveness of the β-carbon to nucleophilic attack, the electrophile needs to have an electron-withdrawing group conjugated with the double bond, such as a nitro, nitrile, or carbonyl group( Ketone, aldehyde and ester).
Reaction Conditions:
The choice of solvent and base (for enolate production) can have a big impact on the reaction. amines, hydroxides, and alkoxides are examples of common bases. The intermediate created during the reaction needs to be stabilized by the solvent. Aprotic polar solvents such as THF, DMF, or DMSO are frequently.
Temperature:
Temperature control is important; too high a temperature can lead to side reactions, while too low a temperature may slow down the reaction.
