Periodic Table
[ez-toc]
The periodic table is the tabular arrangement of elements, according to their atomic number, to study the periodic behavior of their physical and chemical properties. It is a symbol of chemistry but is widely used in physics and all other sciences. It tells us that when elements are arranged in order of increasing atomic number, their properties are repeated. In this article, we will discuss the history, structure, and trends of the periodic table.
 History
History
The history of the periodic table is as follows:
| NAME OF SCIENTIST | YEAR | CONTRIBUTION |
| AL-RAZI | 9th Centaury | Divided all substances into four categories: · Vegetable · Animal · Derivative · Mineral His classification is based on the physical and chemical properties of substances. |
| DOBEREINER | 1829 | Arranged elements known at that time into Triads. |
| JOHN NEWLANDS | 1863-64 | Arranged elements in increasing order of atomic weights. Proposed Law of Octaves |
| LOTHAR MEYER | 1864 | Plotted atomic masses vertically and atomic volumes horizontally to arrange elements. |
| DMITRI MENDELEEV | 1871 | Presented FIRST REGULAR PERIODIC TABLE |
| ANTONIUS VAN DEN BROEK | 1911 | Gave hypothesis of atomic number. |
| HENRY MOSELEY | 1911 | Discovered atomic number experimentally by using X-ray spectroscopy. |
After the discovery of atomic number, the periodic table was rearranged and atomic mass was replaced with atomic number which was a major improvement in Mendeelev’s periodic table and it leads us to the Modern periodic table.
MODERN PERIODIC TABLE
The essential features of the periodic table are given below.
- Groups and periods
- Families
- Blocks
- Metals, Non-metals and Metalloids
GROUPS AND PERIODS
The periodic table is divided into vertical columns called groups and horizontal rows called periods.
Differentiation between the two of them is given below:
| GROUPS | PERIODS |
| Vertical columns in the periodic table | Horizontal rows in the periodic table |
| 8 groups in the periodic table. | 7 rows in the periodic table. |
| Each group is divided into two subgroups i.e A and B | Periods are numbered in Arabic numerals i.e 1 to 7 |
Elements in A subgroups =Normal elements( IA to VIA) Elements in B subgroups =Transition elements( IB to VIIIB) | Elements in periods are arranged as follows: · Period 1=shortest period=2 elements · Period 2 ,3=short periods=8 elements each · Period 4,5=long periods=18 elements each · Period 6,7=very long periods=32 elements in 6th one Ø 7th period is incomplete yet contains 32 elements. |
| Elements in one group possess similar properties. | Elements in one period possess entirely different properties.
|
| Groups are numbered in Roman numerals. | 6th period consists of Lanthanides. |
| 7th period consists of Actinides. |
FAMILIES IN THE PERIODIC TABLE
Following are the families of the periodic table.
| FAMILY NAME | GROUP NO. or ELEMENTS |
| ALKALI METAL | GROUP IA |
| ALKALINE EARTH METAL | GROUP IIA |
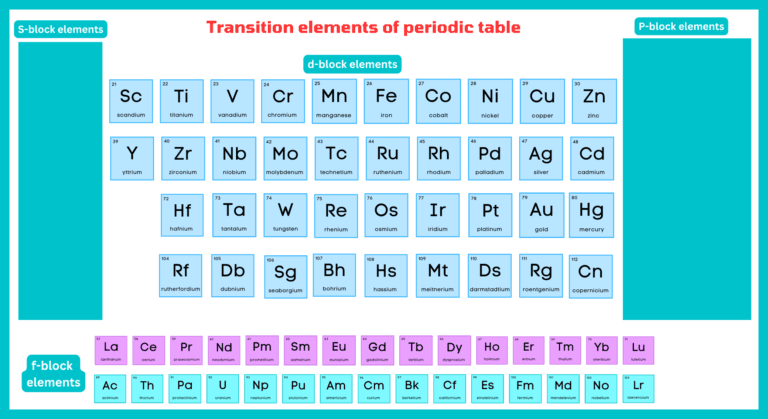
| TRANSITION METALS | GROUP IB to VIIIB |
| NON METALS | Upper elements of GROUP IVA TO VIA |
| HALOGENS | GROUP VIIA |
| NOBLE GASES | GROUP VIIIA |
| METALLOIDS | SOME ELEMENTS OF GROUP IIIA to VIA |
BLOCKS
The periodic table comprises of four blocks.
| s block | Group IA and IIA elements |
| p block | Elements of group IA to VIIIA Except Helium |
| d block | Transition Elements |
| f block | Inner transition elements |
METALS, METALS AND METALLOIDS
A list of metals, nonmetals, and metalloids is given below:
| SUBSTANCE | ELEMENTS |
| METALS | All other elements except non-metals and metalloids |
| NONMETALS | H, He, C, N, P, O, S, Se, F, Cl, Br, I and noble gases(17 elements) |
| METALLOIDS | B, Si, Ge, As, Sb, Te, Po (7 elements) |
TRENDS IN THE PERIODIC TABLE
Chemical properties of elements tend to vary steadily along the group or period. Hence
some common trends of physical properties varying in the periodic table are given below:
| PHYSICAL PROPERTY | GROUPS( TOP TO BOTTOM) | PERIODS(LEFT TO RIGHT) |
| ATOMIC SIZE | INCREASE | DECREASE |
| IONIZATION ENERGY | DECREASE | INCREASE |
| ELECTRON AFFINITY | DECREASE | INCREASE |
| ELECTRONEGATIVITY | DECREASE | INCREASE |
| METALLIC CHARACTER | INCREASE | DECREASE |
 History
History