Definition of Isomerism
The compound has an identical molecular formula but contains different structures and is called isomerism. Here we will discuss the isomerism of carbohydrates.
Types of Isomerism:
- Ketose-aldose isomerism
- D and L isomerism
- Optical isomerism
- Epimerism
- Anomerism
Ketose-aldose isomerism:
Glucose and fructose are isomers of each other, having the same chemical formula, “C6H12O6.”
but they differ in structural formula concerning their functional groups. There is a keto group in position 2 of fructose and an aldehyde group in position 1 of glucose and other examples like glyceraldehyde and dihydroxyacetone. Keep in mind the same chemical (molecular) formula but differ in the functional group. This type of isomerism is known as ketose-aldose isomerism of carbohydrates.

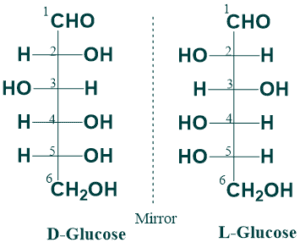
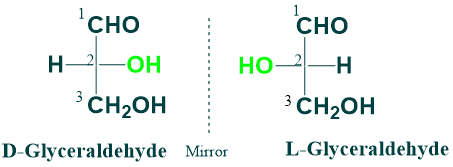
D and L Isomerism:
D and L isomerism depends on the orientation of the H and OH groups around the asymmetric carbon atom adjacent to the primary alcohol group. D and L isomers are mirror images of each other. If the -OH group is on the right side, this sugar belongs to the D-series. If -OH groups are on the left side, this sugar belongs to the L-series.
D and L isomers (enantiomeric pairs) of glucose (at position 5) and glyceraldehyde (at position 2).
Epimerism:
When two monosaccharides differ from each other in their configuration around a single asymmetric carbon atom, they are referred to as epimers of each other.
For example, glucose and galactose are epimers of each other at the 4C position, and glucose and mannose are epimers at the 2C position.
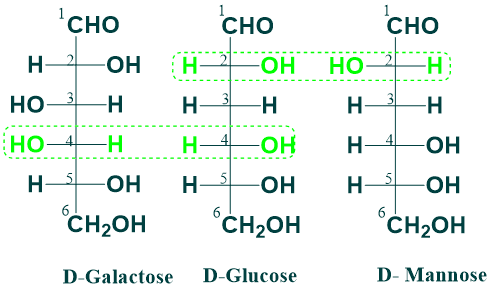
The interconversion of epimers (glucose to galactose and vice versa) is known as epimerization, and a group of enzymes, namely epimerases, catalyze this reaction.
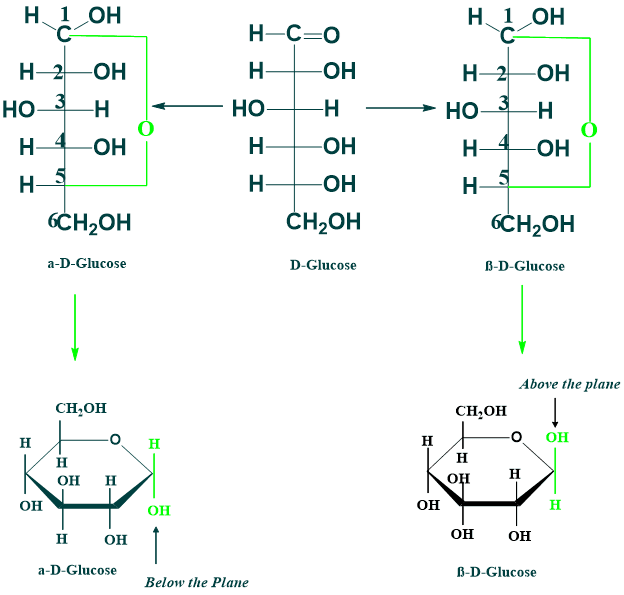
Anomerism:
The predominant form of glucose and fructose in a solution is not in an open chain; the open form of these sugars in a solution cyclizes into rings. The α and β cyclic forms of D-glucose are known as anomers. In the case of the α anomer, in which the hydroxyl group attached to C-1 is below the plane, and in the case of the β anomer, in which the hydroxyl group attached to C-1 is above the plane.