What is chromatography
“Chromatography” is a Greek word that means “separation of pigments, the colored substances.” This word was first used by Russian chemist Mikhail Semenovich Tswett in 1906.
Purpose
It is a technique in which a mixture is converted into its components along a stationary phase by a mobile phase.
phases:
- Stationary phase: it may be solid or liquid.
- Mobile phase: it may be liquid or gas.
Principal:
Absorbing the mixture onto the stationary phase and then the mobile phase allowed it to move across the stationary phase.
Dependence upon:
- properties of stationary and mobile phase
- components of the mixture
- relative retention time or relative retention volume
Retention Time:
Time is required for the mobile phase to hold on to components from the mobile phase.
Retention Volume:
The volume of the mobile phase requires sweeping a component from the stationary phase.
Classification:
Divided into the following categories:
- Liquid Chromatography
- Gas Chromatography
- Column Chromatography
- Paper Chromatography
- Thin Layer Chromatography
- Size Exclusion/Gel Filtration Chromatography
- Ion Exchange Chromatography
- Affinity Chromatography
- Partition Chromatography
- Adsorption Chromatography

Liquid Chromatography:
Chromatography in which the mobile phase is a liquid is called liquid chromatography.
Types of Liquid Chromatography:
- liquid-liquid chromatography
- liquid-solid chromatography
Liquid-Liquid Chromatography:
Liquid chromatography in which the stationary phase is a liquid called liquid chromatography
Liquid-Solid Chromatography:
Liquid chromatography in which the stationary phase is solid called solid-liquid chromatography
- Gas Chromatography:
Chromatography in which the mobile phase is a gas is called gas chromatography.
Types of Gas Chromatography:
- Gas-liquid chromatography
- Gas-solid chromatography
Gas-liquid chromatography:
Chromatography in which the stationary phase is liquid is called gas-liquid chromatography.
Gas-solid chromatography:
Chromatography in which the stationary phase is solid is called gas-solid chromatography.
What is the chromatography used for?
Chromatography is an important biophysical technique that enables the separation, identification, and purification of the components of a mixture for qualitative and quantitative analysis.
What are the 4 steps?
There are 4 steps to a chromatographic analysis:
- sample collection
- sample injection
- sample separation
- sample detection
`What are the uses?
Chromatography plays a vital role in industries we interact with quite often. Pharmaceuticals, clinical trials, environmental and chemical safety, food businesses to separate and analyze additives and beverages, drug testing, forensics, athlete testing, petroleum creation, and molecular biology to check the quality of vitamins, proteins, amino acids, etc. are some of the most common uses of chromatography.
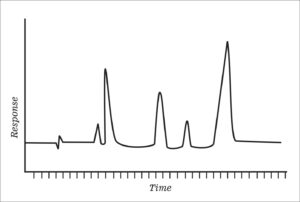
Chromatogram
A visible record (such as a graph) showing the result of separating the components of a mixture by chromatography.