Alkenes
Alkenes are unsaturated compounds having C-C double bonds. They are also called as olefins(meaning “oil forming gas”). Each double- bonded carbons in alkenes is sp2 hybridized .They are reactive as compared to alkanes because of presence of π-bond which is easily broken and hence undergo a number of reactions.
Introduction:
Alkenes are unsaturated hydrocarbons containing C-C double bond having general formula CnH2n, if one C-C double bond is present. If 2 double bonds are present then have general formula CnH2n-2. similarly, if 3 double bonds are present then have general formula CnH2n-3 They are also called as Olefins meaning “Oil forming gas”. They occur abundantly in nature. Ethylene is a plant harmone-induces of ripening in fruits. Similarly, β-Carotene-an alkene having 11 double bonds is a source of Vitamin A and also red color of carrots is because of this alkene.

Alkenes may be Terminal alkenes having double bond at terminal position 0f C- chain; Internal alkenes contain atleast one double bond; Cycloalkenes contain double bond in a ring.
Types of Double Bonds;
Cumulated double bond:
If two double bonds are present at adjacent positions then they are known as cumulated double bonds and this type of alkene is called allene.
For example, CH3CH2CH=C=CH2 (1,2-pentadiene) allene
Conjugated double bond
If two double bonds are seperated by a single bond, then they are called conjugated double bond.
For example, CH3CH=CH-CH=CH2 (1,3- Pentadiene )
Isolated double bonds:
If two double bonds are seperated by two or more single bonds. For, example,
CH2=CH-CH2-CH=CH2 (1,4- pentadiene)
Reactivity of Alkenes Vs Alkanes
Bond dissociation energies of C-C bond in ethane and ethylene can
be used to estimate the strength of pi bond. If we assumed that the bond dissociation energy of C-C sigma bond in ethane and ethylene is same then;
bond dissociation energy of CH2=CH2(σ & π) – bond dissociation energy of CH3-CH3(σ)
635KJ/mol (σ & π) – 368KJ/mol (σ) = 267KJ/mol (π)
As bond dissociation energy of pi bond(267 KJ/mol) is less then as compared to that of sigma ( 368KJ/mol) which means pi bond is much weaker as compared to sigma bond. So, pi bond in case of alkenes is easily broken and it can easily undergo different types of reactions. Hence, alkenes are more reactive as compared to alkanes.
Physical properties of alkenes:
The physical properties of alkenes are similiar to alkanes because of week wander wall forces.
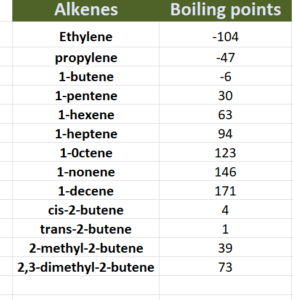
Boiling points:
Like alkanes, boiling points in alkenes increases as no. of carbon atoms increases . This is because high molecular weight alkenes have greater surface area hence intermolecular forces become strong so high temperature is requires to boil substance. But, in case of branched alkenes ,as branching increases boiling points decreases because of reduced surface area. Similarly, boiling points of cis alkenes are higher than trans alkenes as shown in given table;
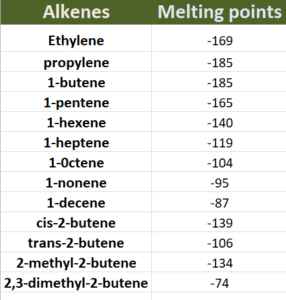
Melting points:
Melting points of alkenes depends not only on no. of carbon atoms but also on packaging of molecules. That’s why branched chain are alkenes have high melting points then normal alkenes of same molecular weight. Melting points of trans alkenes are greater as compared to cis alkenes as shown in given table;
Polarity:
Alkenes are non polar compounds. They are insoluble in water but soluble in non polar solvents like benzene, ether, carbon tertrachloride, choroform etc.
Densities:
Alkenes are less denser than water. But their densities are always slightly higher than those of corresponding alkanes.
Physical States:
At room temperature, lower members of alkenes up to 4 members are gases, the alkenes having 5 to 17 carbon atoms are volatile liquids and those containing more than 18 carbon atoms are solids.
Stability of alkenes:
Stability of alkenes depends on following factors;
- Number of alkyl groups attached to double-bonded carbon atoms
- heats of combustion
- Steric effect
- Hyperconjugation
- position of double bonds
For example

In above examples, heats of combustion of 1-butene is greater which means it is least stable. On the other hand, isobutylene has lowest energy which means it is most stable. The order of stability on the basis of their heats of combustion is as follows;
isobutylene> trans-2-butene > cis-2-butene> 1-butene
This order can be explained on the basis of hperconjugation effect and steric effect as follows;
In 1-butene no steric effect is observed . Similarly , in case of cis 2-butene 2 methyl groups are present on same side of double bond as compared to trans 2-butene in which 2 methyl groups are present on opposite side of double bond . Thus , methyl groups in cis isomer experience greater repulsions as compare to trans alkenes which shows that trans 2-butene is more stable than cis one.
In the same way, hyperconjugation effect in both cis and trans 2- butene is almost same but this effect in 2- butene is still greater then 1-butene. As 2-butene is stable than 1-butene which is proved by hyperconjugation, heats of combustion .
“As no. of alkyl groups on double bonded carbon atoms increases, stability of alkene increases”
Frequently Asked Questions
Why alkenes are also called as Oleifins?
As Oleifins mean oil forming gases , when low molecular weight alkenes which are gases at room temperature react with Bromine or chlorine, they form oily compounds.
Why Cis alkenes have higher boiling points as compared to trans alkenes?
As we know, cis alkenes are polar in nature and have strong intermolecular forces as compared to trans which are non polar in nature. Hence greater energy will be required to boil cis alkenes following higher boiling points as compared to trans alkenes.
Why Trans alkenes have higher melting points as compared to cis alkenes?
Since trans alkenes are highly symmetric in nature as compared to cis alkenes. Hence, molecules of trans alkenes are closely packed and greater amount of energy will be required to melt them following higher melting points as compared to cis alkenes.
